(Dimethylamino)methylene hydantoins as building blocks in the synthesis of oxoaplysinopsins and parabanic acids with antifungal activity†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
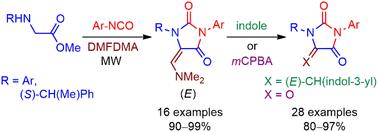
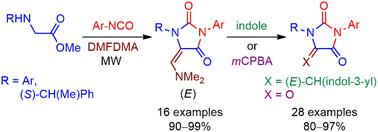
A short, efficient, and stereoselective methodology is described for the synthesis of 5-((dimethylamino)methylene)hydantoins and their conversion into oxoaplysinopsins and parabanic acids. A highly convergent one-pot, two-step reaction between methyl N-arylglycinates, isocyanates, and DMFDMA under microwave irradiation provided the corresponding (dimethylamino)methylene hydantoins as a single E-stereoisomer in high overall yields. The synthesis of (S)-1-(1-phenylethyl) chiral hydantoins, which undergo a stereoselective addition of acetic anhydride, aza-heterocycles, and amines, received special attention. The reaction with indole delivered a series of novel oxoaplysinopsins. Meanwhile, parabanic acids were prepared by a new approach, treating (dimethylamino)methylene hydantoins with mCPBA to generate the oxidative fragmentation of the exocyclic methylene. The antifungal evaluation of the prepared products was carried out on a series of Candida spp., finding potent growth inhibition. According to previous docking studies, this activity is probably due to the inhibitory interaction of the derivatives with the active site of the fungal HMGR enzyme.
将(二甲基氨基)亚甲基海因作为合成具有抗真菌活性的草芹菜素和对位氨基甲酸酯的构建模块
本文介绍了一种简短、高效和立体选择性的方法,用于合成 5-((二甲基氨基)亚甲基)海因并将其转化为氧代芹菜苷元和对位泛酸。在微波辐照下,N-芳基甘氨酸甲酯、异氰酸酯和 DMFDMA 通过高度收敛的一锅两步反应,以单个 E 立体异构体的形式提供了相应的(二甲基氨基)亚甲基海因,总产率高。(S)-1-(1-苯基乙基)手性海因的合成受到了特别关注,它能与乙酸酐、氮杂环和胺发生立体选择性加成反应。与吲哚的反应产生了一系列新型氧代芹菜素。同时,还采用一种新方法制备了对位泛酸,即用 mCPBA 处理(二甲基氨基)亚甲基海因,以产生外环亚甲基的氧化碎片。制备的产品对一系列白色念珠菌属进行了抗真菌评估,发现其具有很强的生长抑制作用。根据之前的对接研究,这种活性可能是由于衍生物与真菌 HMGR 酶的活性位点发生了抑制作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


