Electrochemical sulfonylation/Truce–Smiles rearrangement of N-allylbenzamides: toward sulfone-containing β-arylethylamines and Saclofen analogues†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The β-arylethylamine pharmacophore is commonly found in medications for central nervous system disorders, prompting the need for safe and efficient methods to endow this motif with relevant functional groups for drug discovery. In this context, herein, we have established electrochemical radical sulfonylation reactions of N-allylbenzamides followed by Truce–Smiles rearrangement to produce sulfone- and sulfonate ester-containing β-arylethylamines. Electricity enables this transformation to occur under mild and oxidant-free conditions. Simple sources of sulfonyl radicals and SO2 surrogates were employed to form sulfones and sulfonate esters, respectively. This practical and operationally robust method exhibited a broad substrate scope with good to high yields. The prospective pharmaceutical utility of the process was further demonstrated by removing the N-protecting groups and hydrolysing the sulfonate ester moiety to provide γ-sulfonyl-β-arylamines and Saclofen.
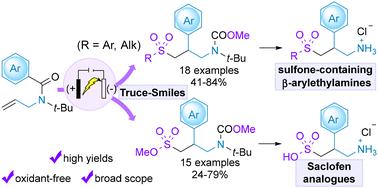
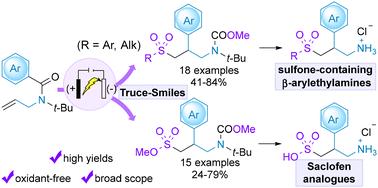
N-烯丙基苯甲酰胺的电化学磺化/云杉-斯迈尔斯重排:向含砜的β-芳基乙胺和沙氯芬类似物发展
在治疗中枢神经系统疾病的药物中,β-芳基乙胺药理结构十分常见,因此需要采用安全高效的方法为这一基团赋予相关官能团,以促进药物发现。在此背景下,我们建立了 N-烯丙基苯甲酰胺的电化学自由基磺酰化反应,然后通过 Truce-Smiles 重排生成含砜和磺酸酯的β-芳基乙胺。电能使这种转化在温和、无氧化剂的条件下进行。利用简单的磺酰基和二氧化硫代用品分别形成砜和磺酸酯。这种实用且操作性强的方法具有广泛的底物范围和良好的产率。通过移除 N 保护基团和水解磺酸酯基团,制备出 γ-磺酰基-β-芳胺和沙氯芬,进一步证明了该方法在制药方面的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


