Sclerostin Antibody-Loaded Dense Collagen Hydrogels Promote Critical-Size Bone Defect Repair
IF 5.4
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
The management of extensive bone loss remains a clinical challenge. Numerous studies are underway to develop a combination of biomaterials, biomolecules, and stem cells to address this challenge. In particular, the systemic administration of antibodies against sclerostin, a regulator of bone formation, was recently shown to enhance the bone repair efficiency of dense collagen hydrogels (DCHs) hosting murine dental pulp stem cells (mDPSCs). The aim of the present study was to assess whether these antibodies, encapsulated and released from DCHs, could promote craniofacial bone repair by the local inhibition of sclerostin. In vitro studies showed that antibody loading modified neither the hydrogel structure nor the viability of seeded mDPSCs. When implanted in a mouse calvaria critical-size bone defect, antibody-loaded DCHs showed repair capabilities similar to those of acellular unloaded DCHs combined with antibody injections. Importantly, the addition of mDPSCs provided no further benefit. Altogether, the local delivery of antisclerostin antibodies from acellular dense collagen scaffolds is highly effective for bone repair. The drastic reduction in the required amount of antibody compared to systemic injection should reduce the cost of the procedure, making the strategy proposed here a promising therapeutic approach for large bone defect repair.
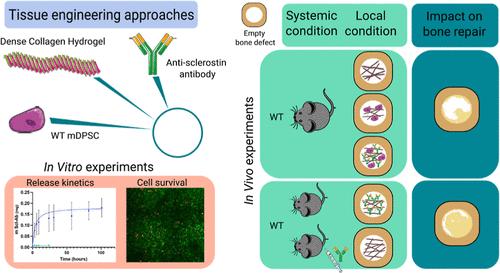
硬骨素抗体负载型致密胶原水凝胶促进临界尺寸骨缺损修复
治疗大面积骨质流失仍是一项临床挑战。目前正在进行大量研究,开发生物材料、生物分子和干细胞的组合,以应对这一挑战。特别是,最近有研究表明,全身注射针对骨形成调节因子硬骨素(sclerostin)的抗体,可提高以小鼠牙髓干细胞(mDPSCs)为宿主的致密胶原水凝胶(DCHs)的骨修复效率。本研究的目的是评估这些从 DCHs 中封装和释放的抗体是否能通过局部抑制硬骨素来促进颅面骨修复。体外研究表明,抗体负载既不会改变水凝胶的结构,也不会改变播种的 mDPSCs 的活力。当植入小鼠小腿临界大小骨缺损时,抗体负载的 DCHs 显示出的修复能力与注射抗体的无细胞 DCHs 相似。重要的是,加入 mDPSCs 并没有带来进一步的益处。总之,从无细胞致密胶原支架局部注射抗硬化剂抗体对骨修复非常有效。与全身注射相比,所需的抗体量大幅减少,这应能降低手术成本,使本文提出的策略成为大面积骨缺损修复的一种很有前景的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Biomaterials Science & Engineering
Materials Science-Biomaterials
CiteScore
10.30
自引率
3.40%
发文量
413
期刊介绍:
ACS Biomaterials Science & Engineering is the leading journal in the field of biomaterials, serving as an international forum for publishing cutting-edge research and innovative ideas on a broad range of topics:
Applications and Health – implantable tissues and devices, prosthesis, health risks, toxicology
Bio-interactions and Bio-compatibility – material-biology interactions, chemical/morphological/structural communication, mechanobiology, signaling and biological responses, immuno-engineering, calcification, coatings, corrosion and degradation of biomaterials and devices, biophysical regulation of cell functions
Characterization, Synthesis, and Modification – new biomaterials, bioinspired and biomimetic approaches to biomaterials, exploiting structural hierarchy and architectural control, combinatorial strategies for biomaterials discovery, genetic biomaterials design, synthetic biology, new composite systems, bionics, polymer synthesis
Controlled Release and Delivery Systems – biomaterial-based drug and gene delivery, bio-responsive delivery of regulatory molecules, pharmaceutical engineering
Healthcare Advances – clinical translation, regulatory issues, patient safety, emerging trends
Imaging and Diagnostics – imaging agents and probes, theranostics, biosensors, monitoring
Manufacturing and Technology – 3D printing, inks, organ-on-a-chip, bioreactor/perfusion systems, microdevices, BioMEMS, optics and electronics interfaces with biomaterials, systems integration
Modeling and Informatics Tools – scaling methods to guide biomaterial design, predictive algorithms for structure-function, biomechanics, integrating bioinformatics with biomaterials discovery, metabolomics in the context of biomaterials
Tissue Engineering and Regenerative Medicine – basic and applied studies, cell therapies, scaffolds, vascularization, bioartificial organs, transplantation and functionality, cellular agriculture
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


