AcOH-Catalyzed Rearrangements of Benzo[e][1,4]diazepin-2(and 3)-ones: Easy Access to 1,4-Dihydroquinazolines and Their Condensed Analogues
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
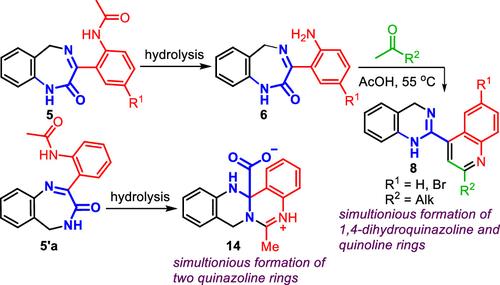
Presented herein is a novel synthesis of new 2-(quinolin-4-yl)-1,4-dihydroquinazoline systems 8, in which the acid-catalyzed rearrangement of spiro[benzo[e][1,4]diazepine-3,4′-quinolin]-2(1H)-ones generated in situ from 3-(2-aminophenyl)-5H-benzo[e][1,4]diazepin-2(1H)-ones 6 with acetone and alkylmethyl ketones has been realized as an important step. An attempt to synthesize isomeric 2-(2-aminophenyl)-5H-benzo[e][1,4]diazepin-3(4H)-one 6′a by hydrolysis of the corresponding N-{2-[5H-benzo[e][1,4]diazepin-3(4H)-on-2-yl]phenyl}acetamide 5′a led to a new heterocyclic system, 6-methyl-8,13-dihydro-13aH-quinazolino[4,3-b]quinazolin-5-ium 13a-carboxylate 14, as a result of an unexpected rearrangement. In addition, it is noteworthy that during the hydrolysis of N-{2-[5H-benzo[e][1,4]diazepin-2(1H)-on-3-yl]phenyl}acetamides 5, the not previously described 14-dihydro-5H-14,5a-(epimino[1,2]benzo)benzo[5,6][1,4]diazepin[2,1-b]quinazolin-6(7H)-ones 7 were unexpectedly obtained.
AcOH 催化的苯并[e][1,4]二氮杂卓-2(和 3)-酮重排:轻松获得 1,4-二氢喹唑啉及其缩合类似物
本文介绍的是一种新的 2-(喹啉-4-基)-1,4-二氢喹唑啉体系 8 的新型合成方法,其中酸催化重排螺[苯并[e][1,4]二氮杂卓-3、4′-喹啉]-2(1H)-酮与丙酮和烷基甲基酮原位生成 3-(2-氨基苯基)-5H-苯并[e][1,4]二氮杂卓-2(1H)-酮 6 的重要步骤。尝试通过水解相应的 N-{2-[5H-苯并[e][1,4]二氮杂卓-3(4H)-酮 6′a,合成异构体 2-(2-氨基苯基)-5H-苯并[e][1,4]二氮杂卓-3(4H)-酮 6′a、4]二氮杂卓-3(4H)-on-2-基]苯基}乙酰胺 5′a,通过水解得到了一个新的杂环体系,即 6-甲基-8,13-二氢-13aH-喹唑啉并[4,3-b]喹唑啉-5-鎓 13a- 羧酸盐 14,这是一个意外重排的结果。此外,值得注意的是,在水解 N-{2-[5H-苯并[e][1,4]二氮杂卓-2(1H)-on-3-基]苯基}乙酰胺 5 的过程中,意外地得到了之前未曾描述过的 14-二氢-5H-14,5a-(表亚氨基[1,2]苯并)苯并[5,6][1,4]二氮杂卓[2,1-b]喹唑啉-6(7H)-酮 7。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


