Low-Cost, Safe, and Anion-Flexible Method for the Electrosynthesis of Diaryliodonium Salts
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
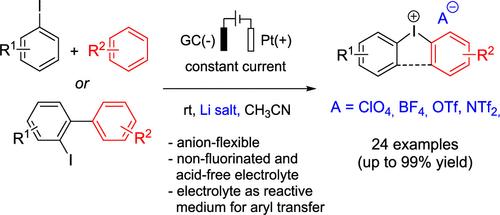
An electrochemical approach toward the synthesis of diaryliodonium salts based on anodic C–I coupling between aryl iodides and arenes is presented. In contrast to previous protocols, our method requires no chemical oxidants, strong acids, or fluorinated solvents. A further advantage is that by use of the appropriate supporting electrolyte, the counterion of choice can be introduced, which is time- and cost-saving as compared to postsynthesis ion exchange. This “anion-flexibility” is particularly interesting when considering the pronounced effect of the counterion on the reactivity of diaryliodonium species in aryl transfer reactions. The scope of our method comprises 24 examples with isolated yields of up to 99%. Scalability was demonstrated by the synthesis on a gram scale. Furthermore, it was shown that the diaryliodonium-containing post-electrolysis solution can be used without further workup as a reactive medium for O-arylation reactions. Finally, a series of para-substituted diaryliodonium compounds was studied using linear sweep voltammetry on a microelectrode and analyzed with respect to the influence of the electronic structure on the redox behavior.
低成本、安全且阴离子灵活的二碘鎓盐电合成方法
本文介绍了一种基于芳基碘化物和炔烃之间阳极 C-I 偶联合成二芳碘鎓盐的电化学方法。与以前的方法相比,我们的方法不需要化学氧化剂、强酸或含氟溶剂。另一个优点是,通过使用适当的支持电解质,可以引入所选择的反离子,与合成后离子交换相比,既节省时间又节约成本。考虑到在芳基转移反应中,反离子对二芳基碘鎓反应活性的明显影响,这种 "阴离子灵活性 "尤为有趣。我们的方法包括 24 个实例,分离产率高达 99%。克级的合成证明了该方法的可扩展性。此外,研究还表明,电解后的含二月桂铵溶液无需进一步处理即可用作 O-芳基化反应的反应介质。最后,利用微电极上的线性扫描伏安法研究了一系列对位取代的二芳碘化合物,并分析了电子结构对氧化还原行为的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


