Rh(III)- or Ru(II)-Catalyzed C–H Annulation with Vinylene Carbonate and an Unexpected Aerobic Oxidation/Deprotection Cascade to Yield Cinnolin-4(1H)-ones
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Transition metal-catalyzed C–H annulation reactions have been extensively utilized for the synthesis of cinnolines, especially the N-protected ones; however, none of them can yield cinnolin-4(1H)-ones, a significant class of bioactive skeletons. Herein, we disclose one-pot access to cinnolin-4(1H)-ones through Rh(III)- or Ru(II)-catalyzed C–H activation/annulation of N-aryl cyclic hydrazides with vinylene carbonate, followed by an O2/K2CO3-promoted aerobic oxidation/deprotection cascade. The π-conjugation of the directing groups plays a crucial role in facilitating this transformation. Notably, seven-membered enolic Rh species IMC is characterized via electrospray ionization mass spectroscopy for the first time, which, along with systematic control experiments, provides compelling evidence for the mechanistic pathway encompassing alkenyl insertion, β-oxygen elimination, protonation, and dehydration.
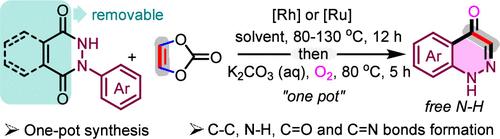
Rh(III)或 Ru(II)-Catalyzed C-H Annulation with Vinylene Carbonate and an Unexpected Aerobic Oxidation/Deprotection Cascade to Yield Cinnolin-4(1H)-ones
过渡金属催化的 C-H 环化反应已被广泛用于合成噌啉类化合物,尤其是 N 保护的噌啉类化合物;然而,这些反应都不能生成噌啉-4(1H)-酮,而噌啉-4(1H)-酮是一类重要的生物活性骨架。在此,我们揭示了通过 Rh(III)- 或 Ru(II)- 催化 N-芳基环酰肼与碳酸乙烯酯的 C-H 活化/annulation,然后通过 O2/K2CO3 促进的有氧氧化/脱保护级联反应,一步法获得噌啉-4(1H)-酮。指导基团的π-共轭在促进这一转化过程中起着至关重要的作用。值得注意的是,首次通过电喷雾离子化质谱鉴定了七元烯醇 Rh 物种 IMC,这与系统控制实验一起,为包括烯基插入、β 氧消除、质子化和脱水的机理途径提供了令人信服的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


