Transgenerational effects of mycorrhiza are stronger in sexual than in clonal offspring of Fragaria vesca and are partly adaptive
IF 5.3
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 0
Abstract

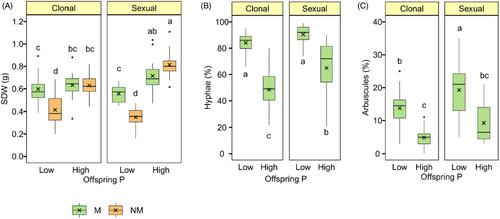
菌根对有性后代的传代效应比对克隆后代的传代效应更强,而且部分是适应性的
这些相互作用是动态的,会因环境条件、植物种类和所涉及的特定真菌种类而有很大不同。例如,当磷(P)或水有限时,植物从菌根中获益的程度要高于这些资源容易获得时(Martínez-García 等人,2012 年;Voříšková 等人,2019 年)。迄今为止发表的为数不多的关于菌根GE的研究表明,与非菌根亲本相比,菌根亲本能培育出性能或体质更好的后代,尤其是在必要资源有限的情况下(Heppell等人,1998年;Koide,2010年;Puy等人,2022年;Varga等人,2013年)。然而,他们并没有分析菌根引发的 TGE 是否具有适应性。克隆后代和有性后代之间菌根引发的 TGE 的程度可能会受到这些繁殖群体之间共生体依赖性潜在差异的进一步影响。这可能是因为克隆植物由于其生长形式,通常有能力水平传播并通过相互连接的柱头直接获取资源,从而减少对真菌共生体获取养分的依赖(Hempel 等人,2013;Onipchenko & Zobel, 2000)。此外,克隆个体还能在零星条件下通过在柱头间共享资源来补偿真菌共生体的服务(Alpert & Stuefer, 1997)。相比之下,有性植物没有这种相互关联的生长习性,它们可能更依赖于菌根真菌来提高养分吸收和抗逆性(Dominiak-Świgoń等人,2021;Fu等人,2010)。这种对菌根关联的不同依赖意味着,菌根亲本通过 TGE 带来的益处可能对有性后代的适应性和表现更为关键。总之,TGE 的某些方面(如克隆繁殖比有性繁殖具有更高的表观遗传保真度)可能会促进克隆后代比有性后代具有更强的 TGE。然而,其他一些因素,如不同生殖起源的个体与菌根共生体之间可能存在的不同关系,表明TGE在有性生殖后代中的生态作用可能比在克隆后代中更强。耐人寻味的是,我们对 TGE 的这些不同方面是否会转化为植物不同繁殖策略之间的功能差异仍缺乏初步了解。在本研究中,我们研究了菌根对克隆草本植物Fragaria vesca的有性后代和克隆后代表现的TGE,比较了菌根对宿主植物(低磷供应量)和对磷营养或生长(高磷供应量)无益的条件。我们假设,根据亲本所经历的特定环境条件,TGE 在克隆后代和有性后代之间的生态和进化意义有所不同。具体来说,我们认为亲本的菌根关系会对有性后代的TGE产生更显著的影响,而非生物条件(如钾的可用性)则会在克隆后代中引发更强的TGE。此外,我们还假设,菌根引起的TGE会受到亲代和子代钾供应量的调节,因为钾供应量会影响资源交换的强度和伙伴间互惠关系的强度。我们还普遍预期 TGE 具有适应性,就像之前报道的非生物条件引起的 TGE 一样(González 等人,2017 年;Yin 等人,2019 年),即如果后代经历了亲本环境与非亲本环境的比较,它们的表现会更好。为了验证这些假设,我们进行了一项对照实验,将亲本植物暴露于不同的菌根和钾供应组合中,并评估亲本和非亲本环境对(a)有性后代的TGE产生的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Ecology
环境科学-生态学
CiteScore
10.90
自引率
5.50%
发文量
207
审稿时长
3.0 months
期刊介绍:
Journal of Ecology publishes original research papers on all aspects of the ecology of plants (including algae), in both aquatic and terrestrial ecosystems. We do not publish papers concerned solely with cultivated plants and agricultural ecosystems. Studies of plant communities, populations or individual species are accepted, as well as studies of the interactions between plants and animals, fungi or bacteria, providing they focus on the ecology of the plants.
We aim to bring important work using any ecological approach (including molecular techniques) to a wide international audience and therefore only publish papers with strong and ecological messages that advance our understanding of ecological principles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


