Unveiling the Cation Dependence in Alkaline Hydrogen Evolution by Differently-Charged Ruthenium/Molybdenum Sulfide Hybrids
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The sluggish kinetics of hydrogen evolution reaction (HER) via water reduction limits the efficiency of alkaline water electrolysis. The HER kinetics is not only intimately related to the catalyst surface structure but also relevant to the cation identity of the electrolyte. The cation dependence also relies on the surface electronic structure and applied potential, but this interrelated effect and its underlying mechanism awaits elucidation. Herein, differently-charged molybdenum sulfide (MoSx) cluster supports ([Mo3S13]2− and [Mo3S7]4+) are utilized to hybridize with the identical metallic Ru centers. The specific electrostatic interaction between MoSx clusters and Ru precursors induces different Ru valences of the hybrids, with a higher valence state for Ru/Mo3S13 endowing a higher activity. The Ru/Mo3S13 and Ru/Mo3S7 exhibited drastically-different cation dependence, in which the charged support determines the local accumulation of cations and resulting water structures. The more negatively-charged Mo3S13 support induces the facile accumulation of cations, especially for less-hydrated K+ cations. The water activation capability by Ru valences and cation accumulation from the support effect in-together determine the cation-dependent alkaline HER activity. This work not only enriches the understanding about the cation-dependent HER mechanism but also shines a light on the rational optimization strategy of electrode/electrolyte interfaces.
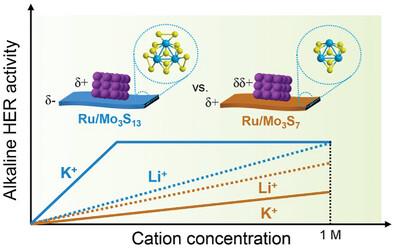
揭示不同电荷的钌/硫化钼杂化物在碱性氢进化过程中的阳离子依赖性
通过还原水进行氢进化反应(HER)的缓慢动力学限制了碱性水电解的效率。氢进化反应动力学不仅与催化剂表面结构密切相关,还与电解质中的阳离子特性有关。阳离子依赖性还依赖于表面电子结构和应用电势,但这种相互关联的效应及其内在机制尚待阐明。本文利用不同电荷的硫化钼(MoSx)簇支撑([Mo3S13]2- 和 [Mo3S7]4+)与相同的金属 Ru 中心杂化。MoSx 团簇与 Ru 前驱体之间的特殊静电作用导致杂化物的 Ru 价态不同,Ru/Mo3S13 的价态越高,活性越高。Ru/Mo3S13 和 Ru/Mo3S7 表现出截然不同的阳离子依赖性,其中带电的支持物决定了阳离子的局部聚集和由此产生的水结构。带负电荷的 Mo3S13 支持物更容易诱导阳离子的积累,尤其是对于水合较少的 K+ 阳离子。Ru 价的水活化能力和支撑效应产生的阳离子积累共同决定了阳离子依赖性碱性 HER 活性。这项工作不仅丰富了人们对阳离子依赖性 HER 机理的理解,还为电极/电解质界面的合理优化策略提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


