A Multifunctional Biomimetic Nanoplatform for Dual Tumor Targeting-Assisted Multimodal Therapy of Colon Cancer
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
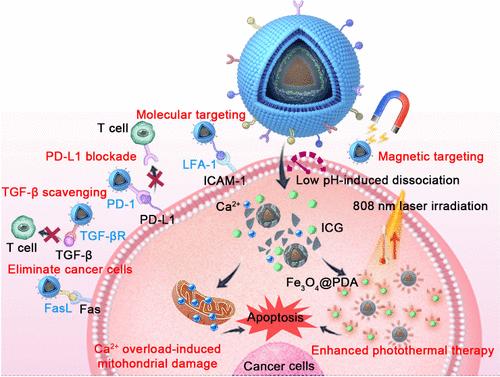
The biomimetic nanoparticles (NPs) possessing abilities of tumor targeting and multimodal therapy show great potential for efficient combat of colon cancer. Herein, we developed a multifunctional biomimetic nanoplatform (Fe3O4@PDA@CaCO3–ICG@CM) based on CaCO3-modified magnetic polydopamine (PDA) loaded with indocyanine green (ICG), which was encapsulated by a mouse lymphoma cell (EL4) membrane (CM) expressing functional proteins (i.e., lymphocyte function-associated antigen 1, LFA-1; transforming growth factor-β receptor, TGF-βR; programmed cell death protein 1, PD-1; and factor related apoptosis ligand, FasL). Under magnetic attraction and LFA-1/PD-1-mediated endocytosis, Fe3O4@PDA@CaCO3–ICG@CM efficiently targeted CT26 colon tumor cells. The released calcium ion (Ca2+) from the NPs triggered by acidic tumor microenvironment, the enhanced photothermal effect contributed by the combination of PDA and ICG, and FasL’s direct killing effect together induced tumor cells apoptosis. Moreover, the apoptosis of CT26 cells induced immunogenic cell death (ICD) to promote the maturation of dendritic cells (DCs) to activate CD4+/CD8+ T cells, thereby fighting against tumor cells, which could further be boosted by programmed death-ligand 1 (PD-L1) blockage and transforming growth factor-β (TGF-β) scavenging by Fe3O4@PDA@CaCO3–ICG@CM. As a result, in vivo satisfactory therapeutic effect was observed for CT26 tumor bearing-mice treated with Fe3O4@PDA@CaCO3–ICG@CM under laser irradiation and magnetic attraction, which could eradicate primary tumors and restrain distant tumors through dual tumor targeting-assisted multimodal therapy and eliciting adaptive antitumor immune response, generating the immune memory for inhibiting tumor metastasis and recurrence. Taken together, the multifunctional biomimetic nanoplatform exhibits superior antitumor effects, providing an insightful strategy for the field of nanomaterial-based treatment of cancer.
用于结肠癌双肿瘤靶向辅助多模式疗法的多功能仿生纳米平台
具有肿瘤靶向和多模式治疗能力的仿生纳米粒子(NPs)在有效防治结肠癌方面具有巨大潜力。在此,我们开发了一种多功能仿生纳米平台(Fe3O4@PDA@CaCO3-ICG@CM),该平台基于负载有吲哚菁绿(ICG)的CaCO3修饰磁性多巴胺(PDA),并由表达功能蛋白(即:淋巴细胞功能相关抗原1,LG-CG)的小鼠淋巴瘤细胞(EL4)膜(CM)包裹、淋巴细胞功能相关抗原 1(LFA-1)、转化生长因子-β 受体(TGF-βR)、程序性细胞死亡蛋白 1(PD-1)和因子相关凋亡配体(FasL)。在磁性吸引和 LFA-1/PD-1 介导的内吞作用下,Fe3O4@PDA@CaCO3-ICG@CM 能有效靶向 CT26 结肠肿瘤细胞。酸性肿瘤微环境诱导 NPs 释放钙离子(Ca2+),PDA 和 ICG 的结合增强了光热效应,FasL 的直接杀伤作用共同诱导肿瘤细胞凋亡。此外,CT26 细胞的凋亡诱导了免疫原性细胞死亡(ICD),从而促进树突状细胞(DCs)的成熟,激活 CD4+/CD8+ T 细胞,进而对抗肿瘤细胞;Fe3O4@PDA@CaCO3-ICG@CM 对程序性死亡配体 1(PD-L1)的阻断和转化生长因子-β(TGF-β)的清除又进一步促进了免疫原性细胞死亡。结果表明,在激光照射和磁场吸引下,用Fe3O4@PDA@CaCO3-ICHG@CM治疗CT26肿瘤小鼠在体内观察到了令人满意的疗效,通过双重肿瘤靶向辅助多模式治疗,可根除原发肿瘤和抑制远处肿瘤,并激发适应性抗肿瘤免疫反应,产生抑制肿瘤转移和复发的免疫记忆。综上所述,多功能仿生纳米平台表现出卓越的抗肿瘤效果,为基于纳米材料的癌症治疗领域提供了一种有远见的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


