Halogenation of Anilines: Formation of Haloacetonitriles and Large-Molecule Disinfection Byproducts
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Aniline-related structures are common in anthropogenic chemicals, such as pharmaceuticals and pesticides. Compared with the widely studied phenolic compounds, anilines have received far less assessment of their disinfection byproduct (DBP) formation potential, even though anilines and phenols likely exhibit similar reactivities on their respective aromatic rings. In this study, a suite of 19 aniline compounds with varying N- and ring-substitutions were evaluated for their formation potentials of haloacetonitriles and trihalomethanes under free chlorination and free bromination conditions. Eight of the aniline compounds formed dichloroacetonitrile at yields above 0.50%; the highest yields were observed for 4-nitroaniline, 3-chloroaniline, and 4-(methylsulfonyl)aniline (1.6–2.3%). Free bromination generally resulted in greater haloacetonitrile yields with the highest yield observed for 2-ethylaniline (6.5%). The trihalomethane yields of anilines correlated with their haloacetonitrile yields. Product analysis of aniline chlorination by liquid chromatography–high-resolution mass spectrometry revealed several large-molecule DBPs, including chloroanilines, (chloro)hydroxyanilines, (chloro)benzoquinone imines, and ring-cleavage products. The product time profiles suggested that the reaction pathways include initial ring chlorination and hydroxylation, followed by the formation of benzoquinone imines that eventually led to ring cleavage. This work revealed the potential of aniline-related moieties in micropollutants as potent precursors to haloacetonitriles and other emerging large-molecule DBPs with the expected toxicity.
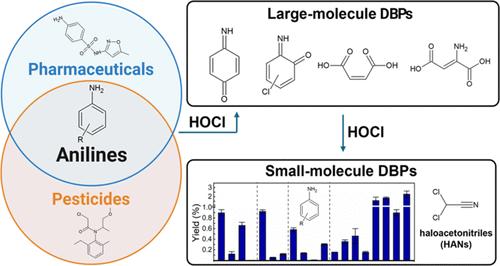
苯胺卤化:卤代乙腈和大分子消毒副产物的形成
苯胺相关结构常见于制药和杀虫剂等人为化学品中。与广泛研究的酚类化合物相比,苯胺类化合物的消毒副产物(DBP)形成潜力评估要少得多,尽管苯胺类化合物和酚类化合物可能在各自的芳香环上表现出相似的反应活性。在这项研究中,我们评估了 19 种具有不同 N 和环取代结构的苯胺化合物在游离氯化和游离溴化条件下形成卤代乙腈和三卤甲烷的潜力。其中八种苯胺化合物形成二氯乙腈的产率超过 0.50%;4-硝基苯胺、3-氯苯胺和 4-(甲磺酰基)苯胺的产率最高(1.6-2.3%)。游离溴化通常会提高卤代乙腈的产率,其中 2-乙基苯胺的产率最高(6.5%)。苯胺的三卤甲烷产率与其卤代乙腈产率相关。通过液相色谱-高分辨质谱法对苯胺氯化反应的产物进行分析,发现了几种大分子 DBPs,包括氯苯胺、(氯)羟基苯胺、(氯)苯醌亚胺和裂环产物。产物时间曲线表明,反应途径包括最初的环氯化和羟基化,然后形成苯醌亚胺,最终导致环裂解。这项工作揭示了微污染物中苯胺相关分子作为卤代乙腈和其他具有预期毒性的新兴大分子 DBP 的有效前体的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


