Formation of a Cathode Electrolyte Interphase on High-Voltage Li-ion Cathodes
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The spinel oxide LiNi0.5Mn1.5O4 (LNMO) currently competes to replace the conventional layered transition metal oxide active material in Li-ion batteries. The high average operating potential (4.8 V vs Li+/Li) challenges the stability of the electrolyte, which, in turn, compromises the lifetime of the Li-ion cell. Online electrochemical mass spectrometry (OEMS) is herein implemented to study the degradation processes occurring at the cathode surface. Gases continuously evolve across subsequent cycles as a result of electrolyte oxidation, a process that is found to be only potentially activated and independent of electrode surface composition. The subsequent formation of protic species autocatalyzes electrolyte salt degradation, which in turn triggers the corrosion of active material, current collector, and conductive carbons. The effectiveness of several well-known electrolyte additives, previously claimed to act as cathode electrolyte interphase (CEI) formers, was explored, revealing the efficacy of phosphorus-based additives. Our study provides a rapid and quantifiable approach to tackle the major challenge of high-voltage cathode materials, namely, their stabilization toward the electrolyte and how to identify and develop an efficient passivating CEI.
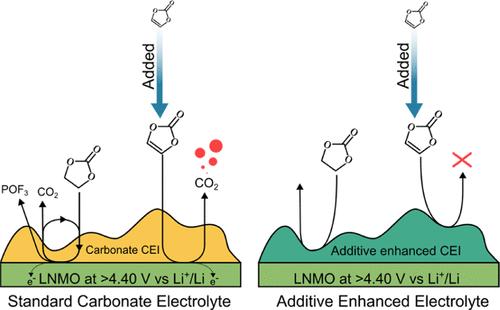
在高压锂离子阴极上形成阴极电解质间质
目前,尖晶石氧化物 LiNi0.5Mn1.5O4(LNMO)正竞相取代锂离子电池中的传统层状过渡金属氧化物活性材料。高平均工作电位(4.8 V vs Li+/Li)对电解质的稳定性提出了挑战,进而影响了锂离子电池的使用寿命。本文采用在线电化学质谱法 (OEMS) 研究阴极表面的降解过程。由于电解质氧化,气体在随后的循环中不断演化,发现这一过程只有潜在的激活作用,且与电极表面成分无关。随后形成的原生物种会自动催化电解质盐降解,进而引发活性材料、集流体和导电碳的腐蚀。我们探索了几种众所周知的电解质添加剂的功效,发现磷基添加剂的功效,这些添加剂以前曾被宣称为阴极电解质相间物(CEI)形成剂。我们的研究提供了一种快速、可量化的方法来解决高压阴极材料面临的主要挑战,即它们对电解质的稳定性以及如何识别和开发高效的钝化 CEI。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


