Targeted protein relocalization via protein transport coupling
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
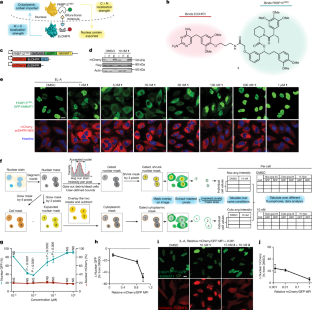
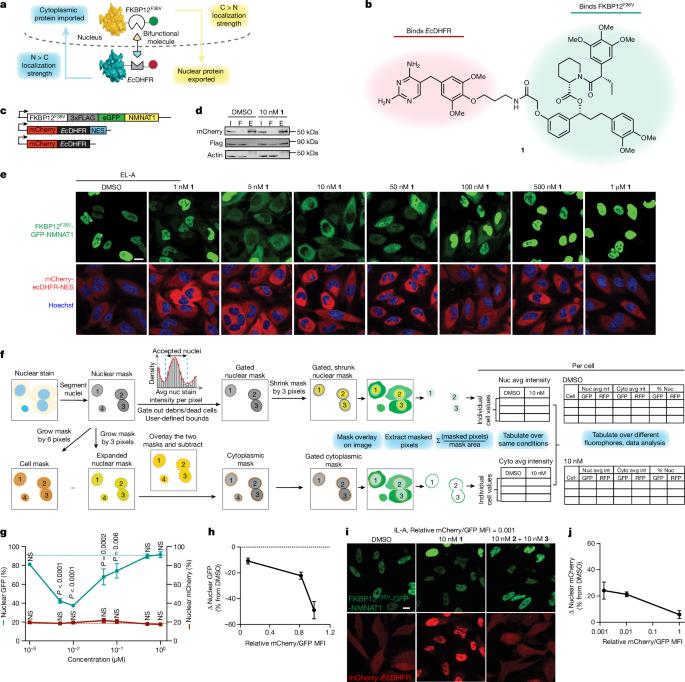
Subcellular protein localization regulates protein function and can be corrupted in cancers1 and neurodegenerative diseases2,3. The rewiring of localization to address disease-driving phenotypes would be an attractive targeted therapeutic approach. Molecules that harness the trafficking of a shuttle protein to control the subcellular localization of a target protein could enforce targeted protein relocalization and rewire the interactome. Here we identify a collection of shuttle proteins with potent ligands amenable to incorporation into targeted relocalization-activating molecules (TRAMs), and use these to relocalize endogenous proteins. Using a custom imaging analysis pipeline, we show that protein steady-state localization can be modulated through molecular coupling to shuttle proteins containing sufficiently strong localization sequences and expressed in the necessary abundance. We analyse the TRAM-induced relocalization of different proteins and then use nuclear hormone receptors as shuttles to redistribute disease-driving mutant proteins such as SMARCB1Q318X, TDP43ΔNLS and FUSR495X. TRAM-mediated relocalization of FUSR495X to the nucleus from the cytoplasm correlated with a reduction in the number of stress granules in a model of cellular stress. With methionyl aminopeptidase 2 and poly(ADP-ribose) polymerase 1 as endogenous cytoplasmic and nuclear shuttles, respectively, we demonstrate relocalization of endogenous PRMT9, SOS1 and FKBP12. Small-molecule-mediated redistribution of nicotinamide nucleotide adenylyltransferase 1 from nuclei to axons in primary neurons was able to slow axonal degeneration and pharmacologically mimic the genetic WldS gain-of-function phenotype in mice resistant to certain types of neurodegeneration4. The concept of targeted protein relocalization could therefore inspire approaches for treating disease through interactome rewiring. Targeted protein relocalization using shuttle proteins with potent ligands amenable to incorporation into targeted relocalization activating molecules could be used to regulate cellular physiology and correct disease states in neurodegenerative diseases, cancer and genetic disorders.
通过蛋白质转运耦合实现有针对性的蛋白质再定位
亚细胞蛋白质定位调控蛋白质功能,在癌症1 和神经退行性疾病2,3 中可能被破坏。重新安排定位以解决疾病驱动表型将是一种极具吸引力的靶向治疗方法。利用穿梭蛋白的迁移来控制目标蛋白亚细胞定位的分子可以实现目标蛋白的重新定位并重新连接相互作用组。在这里,我们发现了一系列具有强效配体的穿梭蛋白,这些配体可以被整合到靶向再定位激活分子(TRAMs)中,并利用这些分子对内源性蛋白质进行再定位。利用定制的成像分析管道,我们证明了蛋白质的稳态定位可以通过与含有足够强的定位序列并以必要的丰度表达的穿梭蛋白的分子耦合来调节。我们分析了 TRAM 诱导的不同蛋白质的重新定位,然后利用核荷尔蒙受体作为穿梭器,重新分配导致疾病的突变蛋白质,如 SMARCB1Q318X、TDP43ΔNLS 和 FUSR495X。在细胞应激模型中,TRAM 介导的 FUSR495X 从细胞质向细胞核的重新定位与应激颗粒数量的减少相关。以蛋氨酰氨基肽酶 2 和聚(ADP-核糖)聚合酶 1 分别作为内源性胞质和核穿梭器,我们证明了内源性 PRMT9、SOS1 和 FKBP12 的重新定位。小分子介导的烟酰胺核苷酸腺苷酸转移酶 1 从原发性神经元的细胞核到轴突的重新分布能够减缓轴突变性,并通过药理作用模拟小鼠的遗传 WldS 功能增益表型,使其对某些类型的神经变性具有抗性4。因此,靶向蛋白质重新定位的概念可以启发通过相互作用组重新布线来治疗疾病的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


