Echocardiographic screening for heart failure and optimization of the care pathway for individuals with pacemakers: a randomized controlled trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
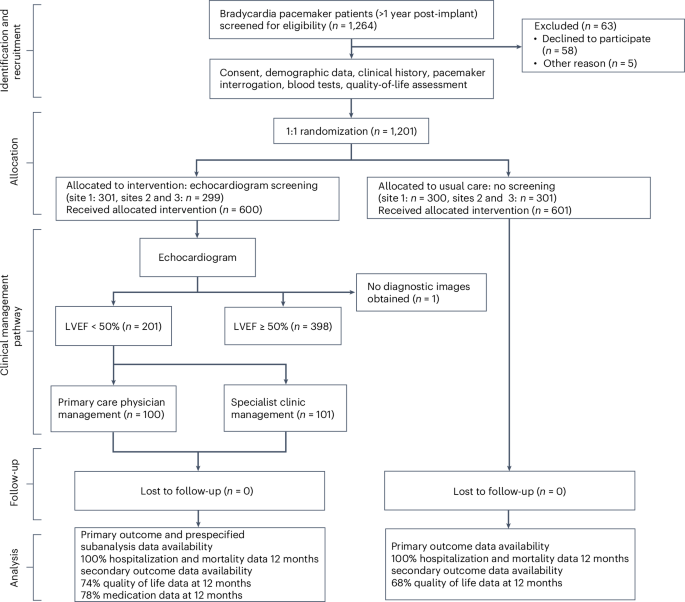
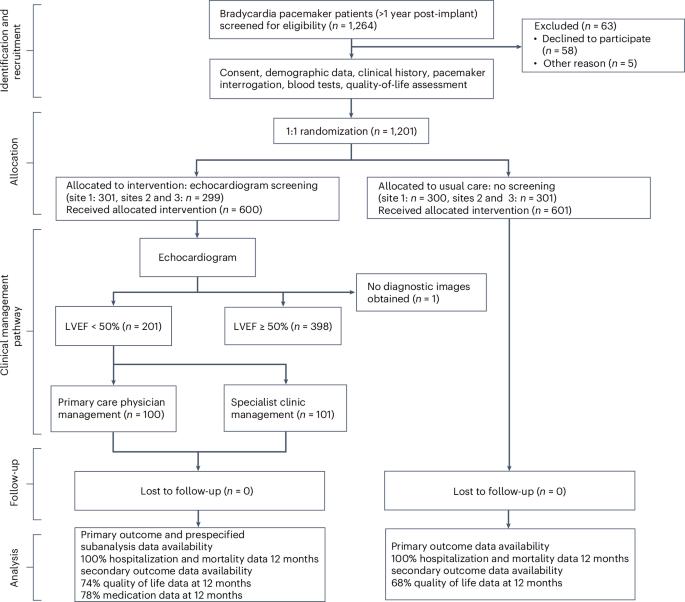
Individuals with pacemakers are at increased risk of left ventricular systolic dysfunction (LVSD). Whether screening for and optimizing the medical management of LVSD in these individuals can improve clinical outcomes is unknown. In the present study, in a multicenter controlled trial (OPT-PACE), we randomized 1,201 patients (717 men) with a pacemaker to echocardiography screening or usual care. In the screening arm, LVSD was detected in 201 of 600 (34%) patients, who then received management in either primary care or a specialist heart failure (HF) and devices clinic. The primary outcome of the trial was the difference in a composite of time to first HF hospitalization or death. Over 31 months (interquartile range = 30–40 months), the primary outcome occurred in 106 of 600 (18%) patients receiving echocardiography screening, which was not significantly different compared with the occurrence of the primary outcome in 115 of 601 (19%) patients receiving the usual care (hazard ratio = 0.89; 95% confidence interval = 0.69, 1.17). In a prespecified, nonrandomized, exploratory analysis, patients with LVSD managed by the specialist clinic experienced the primary outcome event less frequently than those managed in primary care. The results of this trial indicate that echocardiography screening commonly identifies LVSD in individuals with pacemakers but alone does not alter outcomes. ClinicalTrials.gov registration: NCT01819662 . For individuals with pacemakers, a care pathway that includes echocardiographic screening to detect signs of heart failure did not improve cardiac outcomes, but patients flagged as having impaired heart function who were managed by a specialized heart failure clinic benefited, as compared to those managed by primary care physicians.
超声心动图心力衰竭筛查和优化心脏起搏器患者护理路径:随机对照试验
装有心脏起搏器的患者发生左心室收缩功能障碍(LVSD)的风险增加。对这些患者进行左心室收缩功能障碍筛查并优化医疗管理是否能改善临床预后尚不清楚。在本研究中,我们在一项多中心对照试验(OPT-PACE)中对 1201 名安装了心脏起搏器的患者(717 名男性)随机进行了超声心动图筛查或常规治疗。在筛查组中,600 名患者中有 201 人(34%)被检测出 LVSD,他们随后接受了初级保健或心力衰竭(HF)和设备专科门诊的治疗。试验的主要结果是首次心力衰竭住院或死亡时间的综合差异。在 31 个月(四分位间范围 = 30-40 个月)内,接受超声心动图筛查的 600 名患者中有 106 人(18%)出现了主要结果,与接受常规治疗的 601 名患者中有 115 人(19%)出现主要结果相比,差异不大(危险比 = 0.89;95% 置信区间 = 0.69,1.17)。在一项预先指定的非随机探索性分析中,接受专科门诊治疗的 LVSD 患者发生主要结局事件的频率低于接受常规治疗的患者。这项试验的结果表明,超声心动图筛查通常能发现心脏起搏器患者的 LVSD,但仅靠超声心动图筛查并不能改变预后。ClinicalTrials.gov 注册:NCT01819662。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


