Direct cytosolic delivery of siRNA via cell membrane fusion using cholesterol-enriched exosomes
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Efficient cytosolic delivery is a significant hurdle when using short interfering RNA (siRNA) in therapeutic applications. Here we show that cholesterol-rich exosomes are prone to entering cancer cells through membrane fusion, achieving direct cytosolic delivery of siRNA. Molecular dynamics simulations suggest that deformation and increased contact with the target cell membrane facilitate membrane fusion. In vitro we show that cholesterol-enriched milk-derived exosomes (MEs) achieve a significantly higher gene silencing effect of siRNA, inducing superior cancer cell apoptosis compared with the native and cholesterol-depleted MEs, as well as conventional transfection agents. When administered orally or intravenously to mice bearing orthotopic or subcutaneous tumours, the cholesterol-enriched MEs/siRNA exhibit antitumour activity superior to that of lipid nanoparticles. Collectively, by modulating the cholesterol content of exosome membranes to facilitate cell entry via membrane fusion, we provide a promising approach for siRNA-based gene therapy, paving the way for effective, safe and simple gene therapy strategies. Researchers demonstrate that cholesterol-enriched exosomes can deliver siRNA directly into cancer cells, bypassing normal cellular barriers and significantly enhancing gene silencing. This offers a more effective method for gene therapy applications.
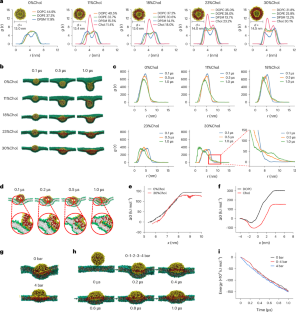

利用富含胆固醇的外泌体通过细胞膜融合直接在细胞膜上递送 siRNA
在治疗应用中使用短干扰 RNA(siRNA)时,高效的胞浆递送是一个重大障碍。在这里,我们展示了富含胆固醇的外泌体容易通过膜融合进入癌细胞,从而实现 siRNA 的直接胞浆递送。分子动力学模拟表明,变形和增加与靶细胞膜的接触有助于膜融合。体外实验表明,富含胆固醇的牛奶衍生外泌体(MEs)可显著提高 siRNA 的基因沉默效果,与原生外泌体、富含胆固醇的外泌体以及传统转染剂相比,可诱导癌细胞凋亡。当小鼠口服或静脉注射富含胆固醇的MEs/siRNA时,其抗肿瘤活性优于脂质纳米颗粒。总之,通过调节外泌体膜的胆固醇含量以促进膜融合进入细胞,我们为基于 siRNA 的基因治疗提供了一种前景广阔的方法,为有效、安全和简单的基因治疗策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


