Nickel-catalyzed directed hydrodefluorination by using water as a hydride source
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We present a new route to construct amides bearing a β,γ-unsaturated gem-difluoroalkene. This amide-directing hydrodefluorination of less reactive CF3-substituted alkenes was achieved by nickel catalysis. In this transformation, water was used as the hydride source. Monodeuterated gem-difluoroalkenes were obtained by using inexpensive deuterium oxide. In addition, the proposed mechanism was supported by both experimental observations and DFT calculation. This protocol complements the existing approaches to expand the diversity of gem-difluoro olefins.
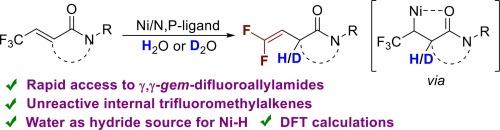
以水为氢化物源的镍催化定向氢氟化反应
我们介绍了一种构建带有 β、γ-不饱和 gem-二氟烯烃的酰胺的新方法。通过镍催化实现了这种活性较低的 CF3 取代烯烃的酰胺定向氢氟化反应。在这一转化过程中,水被用作氢化物源。通过使用廉价的氧化氘,获得了单氘代的宝石-二氟烯烃。此外,实验观察和 DFT 计算都支持所提出的机理。该方案补充了现有的方法,扩大了宝石-二氟烯烃的多样性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


