Covalent immobilization of an enzyme on a layered silicate to catalyze the self-degradation of PCL
Abstract
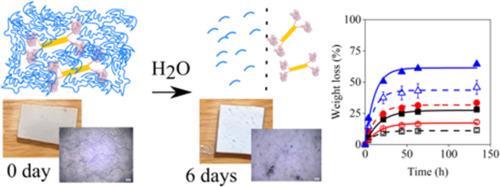
A lipase from Burkholderia cepacia was covalently linked to the surface of Laponite® layered silicate after its activation with glycidoxy moieties on two different routes. The modified silicate was embedded into poly-ε-caprolacton (PCL) for the preparation of self-degradable biopolymers. The activated silicate was characterized by thermogravimetry (TGA) and infrared spectroscopy (FTIR), the location of the linker among the silicate layers was determined by X-ray diffraction (XRD). The activity of the immobilized enzyme was tested in two model reactions, by transesterification in organic medium and hydrolysis in aqueous buffer. The immobilized enzyme was homogenized with the polymer and then films were compression molded at 70 °C. TGA and FTIR measurements verified the successful activation of the silicate but the number of available epoxy groups were limited on the surface. These functional groups linked enzyme molecules to the silicate surface. The enzyme retained its activity even after immobilization and had similar or better catalytic performance than the neat enzyme in both transesterification and hydrolysis. The supported enzyme degraded PCL efficiently, the rate of degradation depended on the type of the linker molecules and on the activated enzyme content of the polymer. The covalently linked enzyme catalyzes the degradation of a solid polymer matrix thus allowing the preparation of self-degradable composites with controlled lifetime and helping the reduction of environmental pollution.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


