Polynitro-1,2,4-triazole Energetic Materials with N-Amino Functionalization
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Trinitromethyl and N-amino groups were innovatively incorporated into the framework of 1,2,4-triazole, resulting in 1-amino-5-nitro-3-(trinitromethyl)-1,2,4-triazole (2). Ammonium and hydrazinium salts of 1-amino-5-nitro-3-(dinitromethyl)-1,2,4-triazole were synthesized by acidification, extraction, and neutralization with bases from the potassium salt. All of the newly prepared energetic compounds were comprehensively characterized by using infrared spectroscopy, elemental analysis, nuclear magnetic resonance spectroscopy, and single crystal X-ray diffraction. Compound 2 exhibits favorable properties such as positive oxygen balance (OBCO2 = 5.8%), high density (1.88 g cm–1), good detonation performances (vD = 8937 m s–1, P = 35.5 GPa), and appropriate friction sensitivity (FS = 144 N). The potassium salt 3 demonstrates good thermal decomposition temperature (181 °C) and high density (1.98 g cm–1), while the ammonium salt and hydrazinium salt also display good thermal decomposition temperatures of 183 and 176 °C, respectively. Among these compounds, the ammonium salt exhibits the lowest mechanical sensitivities (FS = 144 N, IS = 6 J).
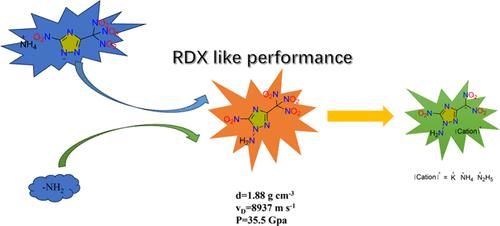
具有 N-氨基官能团的多硝基-1,2,4-三唑高能材料
在 1,2,4- 三氮唑的框架中创新性地加入了三硝基甲基和 N-氨基,从而得到了 1-氨基-5-硝基-3-(三硝基甲基)-1,2,4-三氮唑 (2)。通过对钾盐进行酸化、萃取和碱中和,合成了 1-氨基-5-硝基-3-(二硝基甲基)-1,2,4-三唑的铵盐和肼盐。利用红外光谱、元素分析、核磁共振光谱和单晶 X 射线衍射对所有新制备的高能化合物进行了综合表征。化合物 2 具有正氧平衡(OBCO2 = 5.8%)、高密度(1.88 g cm-1)、良好的引爆性能(vD = 8937 m s-1,P = 35.5 GPa)和适当的摩擦灵敏度(FS = 144 N)等有利特性。钾盐 3 显示出良好的热分解温度(181 ℃)和高密度(1.98 g cm-1),而铵盐和肼盐也分别显示出 183 ℃ 和 176 ℃ 的良好热分解温度。在这些化合物中,铵盐的机械敏感性最低(FS = 144 N,IS = 6 J)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


