Upshifting Lithium Plating Potential To Enhance Electrochemical Lithium Mediated Ammonia Synthesis
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
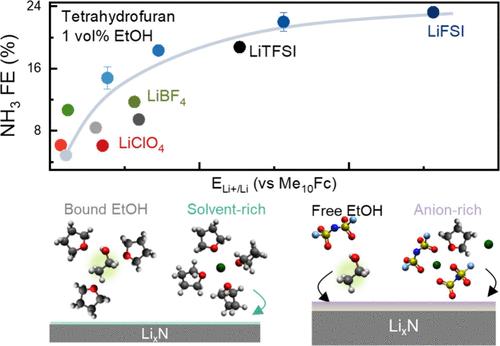
The electrochemical lithium-mediated N2 reduction is a promising process for sustainable ammonia synthesis. Unfortunately, fundamental understanding linking the interfacial chemistry of lithium plating with ammonia efficiency is not well understood. We investigated a series of tetrahydrofuran electrolytes (LiClO4, LiBF4, LiTFSI, LiFSI) at 0.2–7.0 M. The Li+/Li potential (ELi+/Li) measured against the electrolyte-invariant Me10Fc reference increased with more dissociative salts and higher concentration. The upshift in ELi+/Li was found to correlate with greater ammonia production stability and faradaic efficiency as well as the production rate. This correlation could be attributed to altered solid–electrolyte interphase (SEI), which revealed prominent anion-derived (LiF) and alkoxide (LiOEt) species with increasing ELi+/Li from Raman spectroscopy, potentially providing more LixN and enhanced ion transport. Such insights can be used to guide the design of electrolytes to promote lithium-mediated ammonia synthesis for practical applications.
提高镀锂电位以促进电化学锂介导的氨合成
电化学锂介导的 N2 还原是一种很有前景的可持续合成氨工艺。遗憾的是,人们对锂电镀的界面化学与氨效率之间联系的基本认识并不充分。我们研究了一系列 0.2-7.0 M 的四氢呋喃电解质(LiClO4、LiBF4、LiTFSI、LiFSI)。相对于电解质不变的 Me10Fc 参考,测量到的 Li+/Li 电位(ELi+/Li)随着更多的离解盐和更高的浓度而增加。研究发现,ELi+/Li 的上移与更高的氨生产稳定性、远动效率以及生产率相关。拉曼光谱显示,随着 ELi+/Li 的增加,阴离子衍生(LiF)和烷氧基(LiOEt)物种也会增加,这可能会提供更多的 LixN 并增强离子传输。这些见解可用于指导电解质的设计,以促进锂介导的氨合成的实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


