High-Density Atomically Dispersed Metals Activate Adjacent Nitrogen/Carbon Sites for Efficient Ammonia Electrosynthesis from Nitrate
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
While electrocatalytic reduction of nitrate to ammonia presents a sustainable solution for addressing both the environmental and energy issues within the nitrogen cycle, it remains a great challenge to achieve high selectivity and activity due to undesired side reactions and sluggish reaction kinetics. Here, we fabricate a series of metal–N–C catalysts that feature hierarchically ordered porous structure and high-density atomically dispersed metals (HD M1/PNC). Specifically, the as-prepared HD Fe1/PNC catalyst achieves an ammonia production rate of 21.55 mol gcat–1 h–1 that is at least 1 order of magnitude enhancement compared with that of the reported metal–N–C catalysts, while maintaining a 92.5% Faradaic efficiency when run at 500 mA cm–2 for 300 h. In addition to abundant active sites, such high performance benefits from the fact that the high-density Fe can more significantly activate the adjacent N/C sites through charge redistribution for improved water adsorption/dissociation, providing sufficient active hydrogen to Fe sites for nitrate ammoniation, compared with the low-density counterpart. This finding deepens the understanding of high-density metal–N–C materials at the atomic scale and may further be used for designing other catalysts.
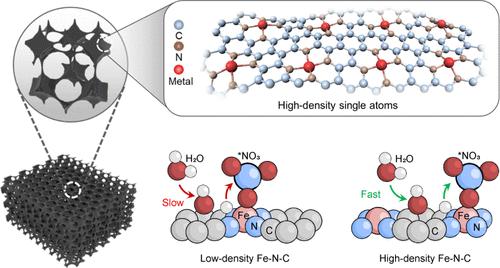
高密度原子分散金属激活相邻氮/碳位点,从硝酸盐中高效电合成氨
电催化将硝酸盐还原为氨是解决氮循环中环境和能源问题的可持续解决方案,但由于不希望发生的副反应和反应动力学迟缓,要实现高选择性和高活性仍是一项巨大挑战。在此,我们制备了一系列具有分层有序多孔结构和高密度原子分散金属(HD M1/PNC)的金属-N-C 催化剂。具体而言,制备的 HD Fe1/PNC 催化剂的氨生产率达到 21.55 mol gcat-1 h-1,与已报道的金属-N-C 催化剂相比至少提高了一个数量级,同时在 500 mA cm-2 下运行 300 h 时仍能保持 92.5% 的法拉第效率。与低密度催化剂相比,除了丰富的活性位点外,这种高性能还得益于高密度铁能通过电荷再分布更显著地激活相邻的 N/C 位点,从而改善水的吸附/解离,为铁位点提供充足的活性氢,以进行硝酸盐氨化。这一发现加深了人们对原子尺度高密度金属-N-C 材料的理解,并可进一步用于设计其他催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


