Sub-2 nm Ternary Metallic Alloy Encapsulated within Montmorillonite Interlayers for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ultrafine metal alloy nanoparticles are emerging as highly effective catalysts for the generation of hydrogen from ammonia borane (AB) hydrolysis. The fabrication of multimetallic alloys with high activity, stability, and metal utilization remains the biggest challenge. Herein, sub-2 nm ternary Rh–Ru–Ni alloys were encapsulated within the interlayers of layer-stripped montmorillonite (MMT) via a simple impregnation method. Experiment and theory results revealed that the synergistic effect of the trimetallic alloy significantly lowers the energy barrier for the AB hydrolysis reaction, by boosting the adsorption and O–H dissociation of H2O molecules. The optimized Rh0.8Ru0.2Ni0.25@MMT-S catalyst achieves high turnover frequency values of 2961 and 784 min–1 at 298 and 273 K, respectively, as well as high recycling stability and thermal resistance. Moreover, the encapsulation method has versatility and can be also applied to synthesize ultrafine Pt- and Ir-based nanoparticles. This study not only highlights the role of the synergistic effect in trimetallic alloys for improving hydrogen evolution but also offers a route to design highly efficient and stable metal nanocatalysts for other applications.
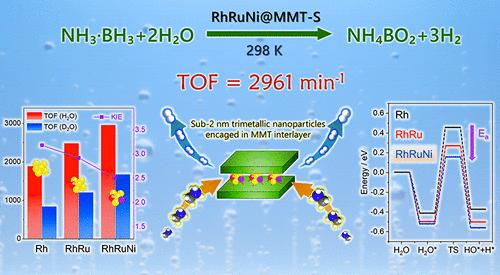
封装在蒙脱石夹层中的亚 2 纳米三元金属合金用于氨硼烷水解高效制氢
超细金属合金纳米粒子正在成为氨硼烷(AB)水解制氢的高效催化剂。如何制造具有高活性、稳定性和金属利用率的多金属合金仍然是最大的挑战。本文通过一种简单的浸渍方法,在层状剥离蒙脱石(MMT)的夹层中封装了亚 2 nm 的三元 Rh-Ru-Ni 合金。实验和理论结果表明,三金属合金的协同效应通过促进 H2O 分子的吸附和 O-H 解离,显著降低了 AB 水解反应的能垒。优化后的 Rh0.8Ru0.2Ni0.25@MMT-S 催化剂在 298 K 和 273 K 条件下分别实现了 2961 和 784 min-1 的高周转频率值,以及较高的回收稳定性和耐热性。此外,该封装方法具有多功能性,还可用于合成超细铂基和铱基纳米颗粒。这项研究不仅强调了三金属合金的协同效应在改善氢气进化方面的作用,而且为设计用于其他应用的高效稳定的金属纳米催化剂提供了一条途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


