Deciphering Asymmetric Brønsted Base-Aminocatalytic Mode in Pudovik/[1,2]-Phospha-Brook Rearrangement/Michael Cascade Reaction
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
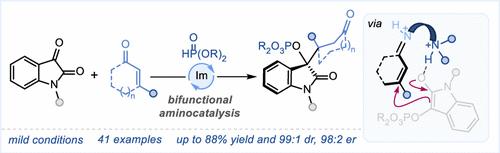
An approach involving the use of a bifunctional aminocatalyst containing Brønsted base and iminium activation sites for asymmetric multicomponent reactions involving [1,2]-phospha-Brook rearrangement has yet to be realized. Herein, we present an aminocatalytic enantioselective conjugate addition of α-phosphonyloxy enolates formed via [1,2]-phospha-Brook rearrangement to α,β-unsaturated ketones. The methodology unfolds a simple one-pot operation consisting of a robust additive-free catalytic system providing a series of oxindole derivatives having two contiguous stereocenters in high yields with excellent stereoselectivities.
解密普多维克/[1,2]-磷杂-布鲁克重排/迈克尔级联反应中的不对称布氏碱-氨基催化模式
在涉及[1,2]-磷-布鲁克重排的不对称多组分反应中,使用含有布氏碱和亚胺活化位点的双功能氨基催化剂的方法尚未实现。在此,我们提出了一种氨基催化对映体选择性共轭加成法,将通过 [1,2]-phospha-Brook 重排形成的 α-磷酰氧基烯醇与 α,β-不饱和酮进行加成。该方法展开了一个简单的一锅操作,由一个强大的无添加剂催化系统组成,提供了一系列具有两个连续立体中心的吲哚衍生物,产量高,立体选择性好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


