Improved Near-Neutral Hydrogen Evolution Reaction Kinetics through Electrolyte Engineering with the Efficient Hydrogen Source NH4+
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A near-neutral HER process suffers from sluggish kinetics. Many efforts have been focused on the design of advanced electrocatalysts. However, the field of electrolyte engineering has rarely been investigated. Considering the complicated ion composition of electrolytes in a near-neutral environment, this work investigated the HER performance of several buffer electrolytes composed of different charged hydrogen sources. The results indicated that a positively charged hydrogen source, namely, NH4+, possessed a superior HER performance to other buffer electrolytes. Under the condition of high concentration, Tafel slopes at 10 and 31 mA·cm–2 were 61 and 84 mV·dec–1, respectively, on the Pt/C catalyst. At an overpotential of 530 mV, the current density of the NH4+ electrolyte was 1000 mA·cm–2 in contrast to only 240 mA·cm–2 for the phosphate buffer solution (PBS) electrolyte. Furthermore, to take a deep perspective into the HER mechanism under a near-neutral environment, based on the experimental values and grand canonical DFT, this work designed a two-step thermodynamic circle to calculate the formation energy of ionic hydrogen sources needed to be transferred from a bulk electrolyte solution to the vicinity of a charged electrode. The result clearly demonstrated that the negatively charged hydrogen sources could not spontaneously approach the Pt electrode surface under certain cathode overpotentials. This work further implemented ab initio molecule dynamics (AIMD) to investigate solvated NH4+ and found that the desolvation process was facilitated by the cathode potential. The proton dissociation process was studied through constrained AIMD. The results clearly showed that the proton dissociated from NH4+ would be directly transferred to the electrode surface, while the proton dissociated from other hydrogen sources would be captured by a hydrogen bond network of water. This discrepancy demonstrated a possibility that NH4+ could directly participate in HER under a near-neutral environment or that the proton dissociation efficiency of NH4+ near the cathode was superior to other hydrogen sources.
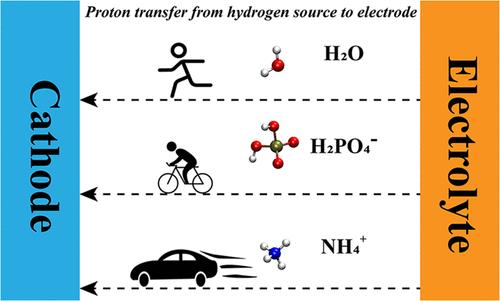
利用高效氢源 NH4+ 通过电解质工程改善近中性氢进化反应动力学
接近中性的 HER 过程存在动力学缓慢的问题。许多人一直致力于设计先进的电催化剂。然而,电解质工程领域却鲜有研究。考虑到近中性环境下电解质中复杂的离子组成,这项研究调查了几种由不同带电氢源组成的缓冲电解质的 HER 性能。结果表明,正电荷氢源 NH4+ 的 HER 性能优于其他缓冲电解质。在高浓度条件下,Pt/C 催化剂在 10 mA-cm-2 和 31 mA-cm-2 时的塔菲尔斜率分别为 61 mV 和 84 mV-dec-1。在 530 mV 的过电位下,NH4+ 电解质的电流密度为 1000 mA-cm-2,而磷酸盐缓冲溶液(PBS)电解质的电流密度仅为 240 mA-cm-2。此外,为了深入探讨近中性环境下的氢氧还原机制,这项研究基于实验值和大规范 DFT,设计了一个两步热力学循环,计算离子氢源从大量电解质溶液转移到带电电极附近所需的形成能。结果清楚地表明,在一定的阴极过电势下,带负电的氢源无法自发地接近铂电极表面。这项工作进一步采用了原子分子动力学(ab initio molecule dynamics,AIMD)来研究溶解的 NH4+,发现阴极电位促进了脱溶过程。通过约束 AIMD 研究了质子解离过程。结果清楚地表明,从 NH4+ 中解离出的质子会直接转移到电极表面,而从其他氢源解离出的质子则会被水的氢键网络捕获。这一差异表明,NH4+ 有可能在接近中性的环境下直接参与 HER,或者 NH4+ 在阴极附近的质子解离效率优于其他氢源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


