A DFT Mechanistic Study on the Aza-Aldol Reaction of Boron Aza-Enolates: Relative Stability of Six-Membered Transition State and Its Relevance to the Coordination Mode of the Leaving Group
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
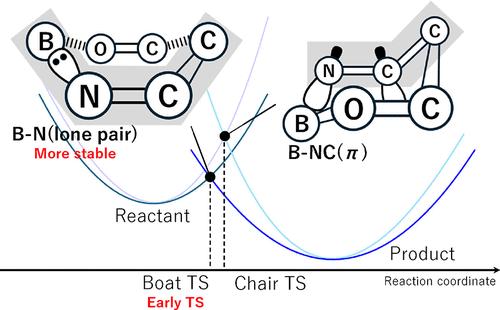
The mechanism of the aza-aldol reaction between boron aza-enolate and benzaldehyde is investigated by using density functional theory calculations. The result shows that the syn-E isomer is preferentially formed, consistent with experimental observations. The six-membered ring transition state (TS) with the boat form leads to the E isomer, while the more unstable chair TS does to the Z isomer. The preference of the syn isomer is determined by the interactions between the substituents of aza-enolate and benzaldehyde. Structural distortion and intrinsic reaction coordinate analyses of simplified model systems provide insights into the origin of the relative stability of the rate-determining TS with boat and chair forms. The boat TS is an early TS; thus, minimal structural distortions of the reactant are required to reach this TS. The Lewis pair interactions between the boron and imine groups during B–N elimination also influenced the relative stability of the TSs. This interaction involves the nitrogen lone pair in the boat TS, while the π(N═C) orbital is involved in the chair TS. The Lewis pair with the lone pair stabilizes the TS more than that with the π orbital. The boron aza-enolate with 9-BBN generates an ate complex and forms C–C bonds sequentially, whereas that with Bpin does not generate an ate complex and exhibits the concerted formation of B–O and C–C bonds. Thus, the higher electrophilicity of boron such as 9-BBN enhances the reactivity by facilitating the formation of the ate complex. A reaction design is proposed to reverse the syn/anti selectivity. Proof-of-concept DFT calculations suggested that the modification of the imine group would change the relative stability of the boat/chair TSs and give the anti-product.
硼氮杂环戊醇的氮杂环-醛醇反应的 DFT 力学研究:六元过渡态的相对稳定性及其与离去基团配位模式的相关性
利用密度泛函理论计算研究了氮杂烯酸硼和苯甲醛之间的氮杂醛反应机理。结果表明,合成-E 异构体优先形成,这与实验观察结果一致。六元环过渡态(TS)的舟状形式会导致 E 异构体,而更不稳定的椅状 TS 则会导致 Z 异构体。合成异构体的偏好是由氮杂烯酸和苯甲醛的取代基之间的相互作用决定的。通过对简化模型系统的结构畸变和内在反应坐标分析,可以深入了解决定速率的舟形和椅形 TS 相对稳定性的来源。舟状 TS 是一种早期 TS;因此,要达到这种 TS,反应物的结构畸变最小。在 B-N 消去过程中,硼和亚胺基团之间的路易斯对相互作用也会影响 TS 的相对稳定性。在舟状 TS 中,这种相互作用涉及氮孤对;而在椅状 TS 中,则涉及 π(N═C)轨道。具有孤对的路易斯对比具有π轨道的路易斯对更能稳定TS。与 9-BBN 结合的偶氮烯酸硼会生成ate 复合物,并依次形成 C-C 键,而与 Bpin 结合的偶氮烯酸硼则不会生成ate 复合物,而是协同形成 B-O 键和 C-C 键。因此,硼(如 9-BBN)的亲电性较高,可促进ate 复合物的形成,从而提高反应活性。我们提出了一种逆转合成/反选择性的反应设计。概念验证 DFT 计算表明,对亚胺基团的修饰会改变舟/椅 TS 的相对稳定性,并产生反产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


