Specificity of Membrane-Associated J-Domain Protein, Caj1, in Amphotericin B Tolerance in Budding Yeast
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hsp70:J-domain protein (JDP) machineries play pivotal roles in maintaining cellular proteostasis and governing various aspects of fungal physiology. While Hsp70 is known for its involvement in conferring tolerance to diverse antifungal drugs, the specific contribution of JDPs remains unclear. In this study, we examined the sensitivity of cytosolic JDP deletion strains of budding yeast to amphotericin B (AmB), a polyene antifungal agent widely utilized in fungal disease treatment due to its ability to disrupt the fungal plasma membrane (PM). Deleting Caj1, a PM-associated class II JDP, heightened susceptibility to AmB, and the protection conferred by Caj1 against AmB necessitated both its N-terminal J-domain and C-terminal lipid binding domain. Moreover, Caj1 deficiency compromised PM integrity as evidenced by increased phosphate efflux and exacerbated AmB sensitivity, particularly at elevated temperatures. Notably, phytosphingosine (PHS) addition as well as overexpression of PMP3, a positive PM integrity regulator, significantly rescued AmB sensitivity of caj1Δ cells. Our results align with the notion that Caj1 associates with the PM and cooperates with Hsp70 to regulate PM proteostasis, thereby influencing PM integrity in budding yeast. Loss of Caj1 function at the PM compromises PM protein quality control, thereby rendering yeast cells more susceptible to AmB.
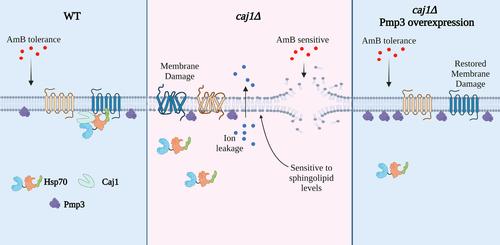
膜相关 J-结构域蛋白 Caj1 在芽殖酵母耐受两性霉素 B 过程中的特异性
Hsp70:J-结构域蛋白(JDP)机制在维持细胞蛋白稳态和管理真菌生理的各个方面发挥着关键作用。众所周知,Hsp70 参与赋予真菌对多种抗真菌药物的耐受性,但 JDPs 的具体贡献仍不清楚。在这项研究中,我们检测了芽殖酵母细胞膜 JDP 缺失菌株对两性霉素 B(AmB)的敏感性,两性霉素 B 是一种多烯类抗真菌药,因其具有破坏真菌质膜(PM)的能力而被广泛用于真菌疾病的治疗。删除与 PM 相关的 II 类 JDP Caj1 会增加对 AmB 的敏感性,而 Caj1 对 AmB 的保护作用需要其 N 端 J 域和 C 端脂质结合域。此外,Caj1 的缺乏会损害 PM 的完整性,表现为磷酸盐外流增加和对 AmB 的敏感性加剧,尤其是在温度升高时。值得注意的是,植物鞘磷脂(PHS)的添加以及PMP3(一种积极的PM完整性调节因子)的过表达能显著缓解caj1Δ细胞对AmB的敏感性。我们的研究结果与以下观点一致:Caj1与PM结合,并与Hsp70合作调节PM的蛋白稳态,从而影响芽殖酵母中PM的完整性。失去 Caj1 在 PM 上的功能会影响 PM 蛋白质的质量控制,从而使酵母细胞更容易受到 AmB 的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


