Energy Recovery from Hexavalent Chromium Reduction for In Situ Electrocatalytic Hydrogen Peroxide Production
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
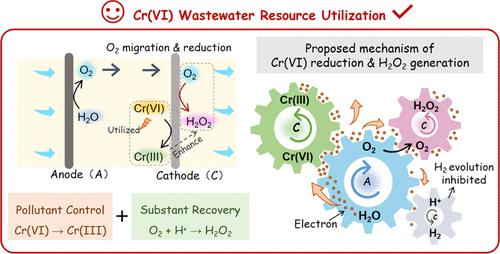
Recovering chemical energy embedded in pollutants is significant in achieving carbon-neutral industrial wastewater treatment. Considering that industrial wastewater is usually treated in a decentralized manner, in situ utilization of chemical energy to achieve waste-to-treasure should be given priority. Herein, the chemical energy released by the electroreduction of Cr(VI) was used to enhance on-site H2O2 generation in a stacked flow-through electrochemical system. The driving force of water flow efficiently coupled O2 evolution with 2-e O2 reduction to facilitate H2O2 generation by transporting anode-produced O2 to the cathode. Meanwhile, the chemical energy released by Cr(VI) promoted O2 evolution and impeded H2 evolution by regulating the electrode potentials, accounting for the enhanced H2O2 generation. The system could completely reduce 10–100 ppm of Cr(VI), reaching the maximum H2O2 concentration of 2.41 mM. In particular, the H2O2 concentrations in the Cr(VI)-containing electrolyte were 10.6–88.1% higher than those in the Cr(VI) free electrolyte at 1.8–2.5 V. A 24-day continuous experiment demonstrated the high efficiency and stability of the system, achieving a 100% reduction efficiency for 100 ppm of Cr(VI) and producing ∼1.5 mM H2O2 at 1.8 V. This study presents a feasible strategy for Cr(VI) detoxification and synchronous on-site H2O2 generation, providing a new perspective for innovative Cr(VI) wastewater treatment toward resource utilization.
原位电催化过氧化氢生产中六价铬还原的能量回收
回收污染物中蕴含的化学能对实现碳中和的工业废水处理意义重大。考虑到工业废水通常以分散方式处理,因此应优先考虑就地利用化学能实现变废为宝。在此,我们利用 Cr(VI) 电还原释放的化学能,在叠加式直流电化学系统中提高 H2O2 的现场生成。水流的驱动力有效地将 O2 演化与 2-e O2 还原耦合在一起,通过将阳极产生的 O2 运输到阴极来促进 H2O2 的生成。同时,六价铬释放的化学能通过调节电极电位促进了 O2 的进化,阻碍了 H2 的进化,从而提高了 H2O2 的生成。该系统可以完全还原 10-100 ppm 的六(六)铬,达到 2.41 mM 的最大 H2O2 浓度。特别是,在 1.8-2.5 V 的电压下,含六价铬电解液中的 H2O2 浓度比不含六价铬电解液中的 H2O2 浓度高 10.6-88.1%。为期 24 天的连续实验证明了该系统的高效性和稳定性,对 100 ppm 的六(六)铬的还原效率达到了 100%,并在 1.8 V 电压下产生了 ∼1.5 mM 的 H2O2。该研究提出了一种可行的六(六)铬解毒和现场同步生成 H2O2 的策略,为实现资源化利用的创新六(六)铬废水处理提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


