Fused radical SAM and αKG-HExxH domain proteins contain a distinct structural fold and catalyse cyclophane formation and β-hydroxylation
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Two of nature’s recurring binding motifs in metalloproteins are the CxxxCxxC motif in radical SAM enzymes and the 2-His-1-carboxylate motif found both in zincins and α-ketoglutarate and non-haem iron enzymes. Here we show the confluence of these two domains in a single post-translational modifying enzyme containing an N-terminal radical S-adenosylmethionine domain fused to a C-terminal 2-His-1-carboxylate (HExxH) domain. The radical SAM domain catalyses three-residue cyclophane formation and is the signature modification of triceptides, a class of ribosomally synthesized and post-translationally modified peptides. The HExxH domain is a defining feature of zinc metalloproteases. Yet the HExxH motif-containing domain studied here catalyses β-hydroxylation and is an α-ketoglutarate non-haem iron enzyme. We determined the crystal structure for this HExxH protein at 2.8 Å, unveiling a distinct structural fold, thus expanding the family of α-ketoglutarate non-haem iron enzymes with a class that we propose to name αKG-HExxH. αKG-HExxH proteins represent a unique family of ribosomally synthesized and post-translationally modified peptide modifying enzymes that can furnish opportunities for genome mining, synthetic biology and enzymology. Radical SAM (rSAM) and 2-His-1-carboxylate enzymes are known to co-occur in RiPP biosynthetic pathways. Here we show the fusion of these enzymes in a single protein where the rSAM enzyme catalyzes cyclophane formation. The 2-His-1-carboxylate enzymes—termed αKG-HExxH—are α-ketoglutarate non-haem iron enzymes that harbour a distinct fold and catalyse β-hydroxylation.
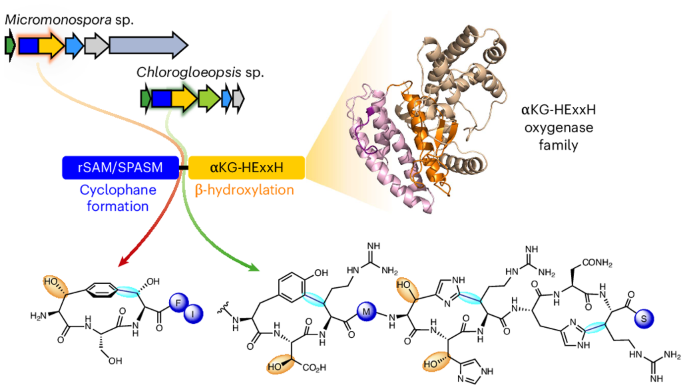
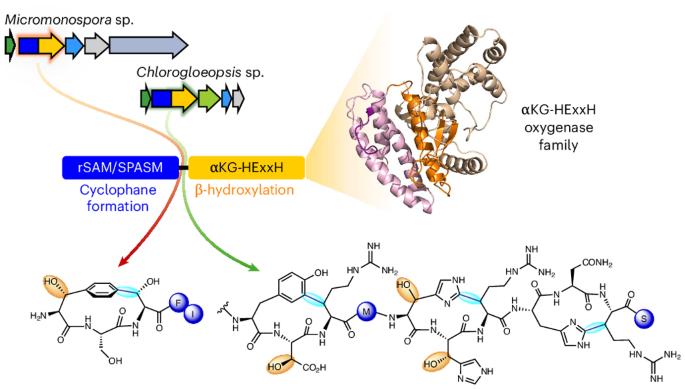
融合自由基 SAM 和 αKG-HExxH 结构域蛋白含有不同的结构折叠,可催化环烷形成和 β- 羟基化作用
在金属蛋白中,有两个自然界反复出现的结合基团,一个是自由基 SAM 酶中的 CxxxCxxC 基团,另一个是在锌蛋白和 α-酮戊二酸及非血铁酶中发现的 2-His-1-羧酸基团。在这里,我们展示了这两个结构域在一个单一的翻译后修饰酶中的融合,该酶包含一个 N 端自由基 S-腺苷蛋氨酸结构域和一个 C 端 2-His-1-羧酸(HExxH)结构域。自由基 SAM 结构域催化三残基环烷的形成,是三肽这种由核糖体合成并经翻译后修饰的肽类的标志性修饰。HExxH 结构域是锌金属蛋白酶的一个显著特征。然而,本文研究的含 HExxH 主题结构域可催化β-羟基化反应,是一种α-酮戊二酸非血铁酶。我们测定了这种 HExxH 蛋白 2.8 Å 的晶体结构,揭示了一个独特的结构折叠,从而扩展了α-酮戊二酸非血红素铁酶家族,我们建议将其命名为αKG-HExxH。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


