A cell-autonomous role for border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
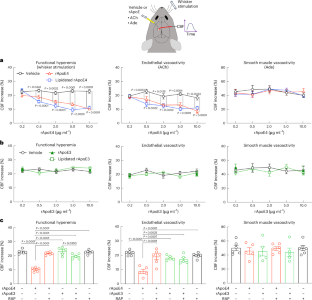
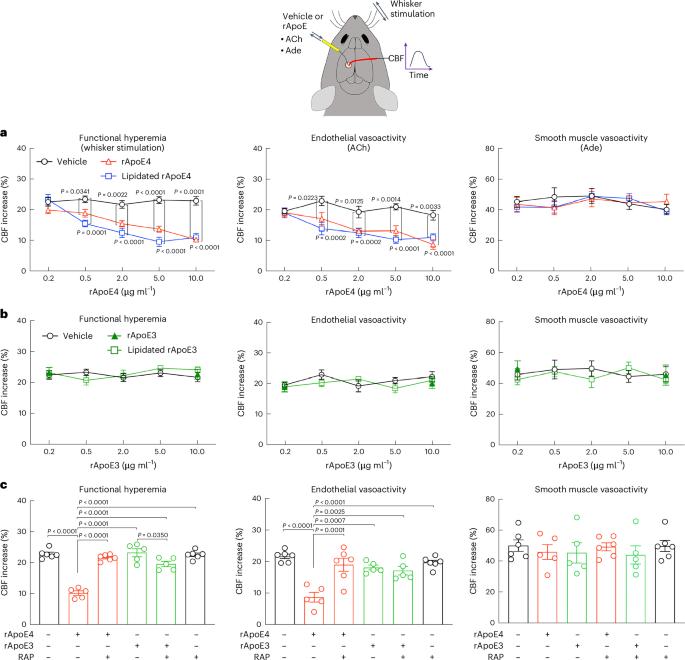
Apolipoprotein E4 (ApoE4), the strongest genetic risk factor for sporadic Alzheimer’s disease, is also a risk factor for microvascular pathologies leading to cognitive impairment, particularly subcortical white matter injury. These effects have been attributed to alterations in the regulation of the brain blood supply, but the cellular source of ApoE4 and the underlying mechanisms remain unclear. In mice expressing human ApoE3 or ApoE4, we report that border-associated macrophages (BAMs), myeloid cells closely apposed to neocortical microvessels, are both sources and effectors of ApoE4 mediating the neurovascular dysfunction through reactive oxygen species. ApoE4 in BAMs is solely responsible for the increased susceptibility to oligemic white matter damage in ApoE4 mice and is sufficient to enhance damage in ApoE3 mice. The data unveil a new aspect of BAM pathobiology and highlight a previously unrecognized cell-autonomous role of BAM in the neurovascular dysfunction of ApoE4 with potential therapeutic implications. ApoE4 is a risk factor for Alzheimer’s disease and vascular dementia. We report that in ApoE4 mice perivascular macrophages are the sole source and effectors of the ApoE4 mediating the neurovascular dysfunction, enhanced white matter damage and cognitive impairment.
边界相关巨噬细胞在载脂蛋白E4神经血管功能障碍和白质损伤易感性中的细胞自主作用
载脂蛋白 E4(ApoE4)是散发性阿尔茨海默病的最强遗传风险因素,也是导致认知障碍的微血管病变的风险因素,尤其是皮层下白质损伤。这些影响被归因于大脑供血调节的改变,但载脂蛋白E4的细胞来源和内在机制仍不清楚。在表达人类载脂蛋白E3或载脂蛋白E4的小鼠中,我们发现边界相关巨噬细胞(BAMs)--与新皮质微血管紧密相连的髓细胞--既是载脂蛋白E4的来源,也是载脂蛋白E4的效应器,通过活性氧介导神经血管功能障碍。BAMs 中的载脂蛋白 E4 是导致载脂蛋白 E4 小鼠白质少血症损伤易感性增加的唯一原因,也足以加重载脂蛋白 E3 小鼠的损伤。这些数据揭示了 BAM 病理生物学的一个新方面,并强调了 BAM 在载脂蛋白 E4 神经血管功能障碍中的一个以前未被认识到的细胞自主作用,具有潜在的治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


