Synergistic association of Aβ and tau pathology with cortical neurophysiology and cognitive decline in asymptomatic older adults
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
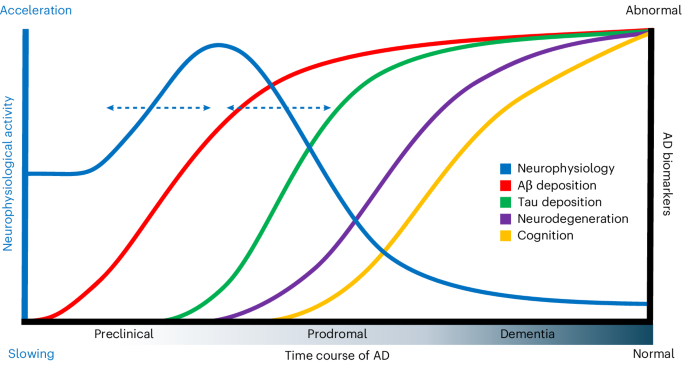
Animal and computational models of Alzheimer’s disease (AD) indicate that early amyloid-β (Aβ) deposits drive neurons into a hyperactive regime, and that subsequent tau depositions manifest an opposite, suppressive effect as behavioral deficits emerge. Here we report analogous changes in macroscopic oscillatory neurophysiology in the human brain. We used positron emission tomography and task-free magnetoencephalography to test the effects of Aβ and tau deposition on cortical neurophysiology in 104 cognitively unimpaired older adults with a family history of sporadic AD. In these asymptomatic individuals, we found that Aβ depositions colocalize with accelerated neurophysiological activity. In those also presenting medial–temporal tau pathology, linear mixed effects of Aβ and tau depositions indicate a shift toward slower neurophysiological activity, which was also linked to cognitive decline. We conclude that early Aβ and tau depositions relate synergistically to human cortical neurophysiology and subsequent cognitive decline. Our findings provide insight into the multifaceted neurophysiological mechanisms engaged in the preclinical phases of AD. Gallego-Rudolf et al. report accelerated brain activity with initial amyloid-β deposition in asymptomatic individuals. In those where tau also starts accumulating, brain activity decelerates, correlating with subsequent cognitive decline.
无症状老年人的 Aβ 和 tau 病理学与皮层神经生理学和认知能力下降之间的协同关系
阿尔茨海默病(AD)的动物模型和计算模型表明,早期淀粉样蛋白-β(Aβ)沉积会促使神经元进入亢奋状态,而随后的tau沉积则会随着行为障碍的出现而产生相反的抑制作用。在这里,我们报告了人脑中宏观振荡神经生理学的类似变化。我们使用正电子发射断层扫描和无任务脑磁图来测试 Aβ 和 tau 沉积对 104 名有散发性老年痴呆症家族史、认知功能未受损的老年人大脑皮层神经生理学的影响。我们发现,在这些无症状的人中,Aβ沉积与神经电生理活动的加速有共同之处。在那些同时出现内颞侧 tau 病理学的患者中,Aβ 和 tau 沉积的线性混合效应表明神经生理活动转向缓慢,这也与认知能力下降有关。我们的结论是,早期 Aβ 和 tau 沉积与人类大脑皮层神经生理学和随后的认知能力下降有协同关系。我们的研究结果让我们深入了解了多发性硬化症临床前期所涉及的多方面神经生理学机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


