Fundamental Insights into the Direct Electron Transfer Mechanism on Ag Atomic Cluster
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The nonradical oxidation pathway for pollutant degradation in Fenton-like catalysis is favorable for water treatment due to the high reaction rate and superior environmental robustness. However, precise regulation of such reactions is still restricted by our poor knowledge of underlying mechanisms, especially the correlation between metal site conformation of metal atom clusters and pollutant degradation behaviors. Herein, we investigated the electron transfer and pollutant oxidation mechanisms of atomic-level exposed Ag atom clusters (AgAC) loaded on specifically crafted nitrogen-doped porous carbon (NPC). The AgAC triggered a direct electron transfer (DET) between the terminal oxygen (Oα) of surface-activated peroxodisulfate and the electron-donating substituents-containing contaminants (EDTO–DET), rendering it 11–38 times higher degradation rate than the reported carbon-supported metal catalysts system with various single-atom active centers. Heterocyclic substituents and electron-donating groups were more conducive to degradation via the EDTO–DET system, while contaminants with high electron-absorbing capacity preferred the radical pathway. Notably, the system achieved 79.5% chemical oxygen demand (COD) removal for the treatment of actual pharmaceutical wastewater containing 1053 mg/L COD within 30 min. Our study provides valuable new insights into the Fenton-like reactions of metal atom cluster catalysts and lays an important basis for revolutionizing advanced oxidation water purification technologies.
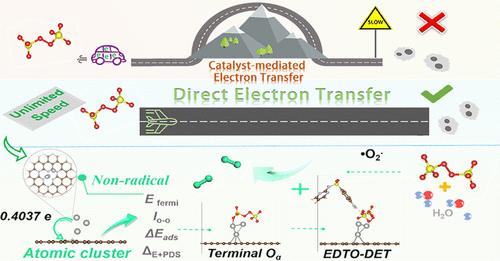
对银原子团簇上直接电子转移机制的基本认识
Fenton 类催化中污染物降解的非自由基氧化途径具有反应速率高、环境稳健性好等优点,有利于水处理。然而,由于我们对其基本机理知之甚少,尤其是对金属原子簇的金属位点构象与污染物降解行为之间的相关性知之甚少,因此此类反应的精确调控仍然受到限制。在此,我们研究了负载在特制的掺氮多孔碳(NPC)上的原子级暴露的银原子簇(AgAC)的电子传递和污染物氧化机制。AgAC 触发了表面活性过硫酸根的末端氧(Oα)与含电子捐赠取代基污染物之间的直接电子转移(DET)(EDTO-DET),使其降解率比已报道的具有各种单原子活性中心的碳支撑金属催化剂体系高出 11-38 倍。杂环取代基和电子捐赠基团更有利于通过 EDTO-DET 系统进行降解,而具有高电子吸收能力的污染物则更倾向于自由基途径。值得注意的是,该系统在 30 分钟内处理实际含有 1053 mg/L COD 的制药废水时,化学需氧量(COD)的去除率达到 79.5%。我们的研究为金属原子团簇催化剂的芬顿类反应提供了宝贵的新见解,为革新高级氧化水净化技术奠定了重要基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


