Chemosynthetic P4HB: A Ten-Year Journey from a “Non-Polymerizable” Monomer to a High-Performance Biomaterial
IF 14
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
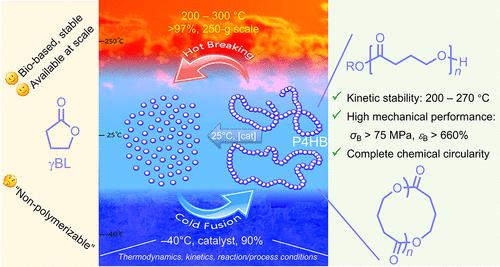
Aliphatic polyesters consisting of hydrolytically and/or enzymatically degradable ester bonds in each repeating unit possess diverse thermomechanical properties and desired biodegradability and biocompatibility, thus, finding broad applications in biomedical fields. Among them, poly(4-hydroxybutyrate) (P4HB) is a biomaterial receiving particular attention, due to its proper thermal transition temperatures (Tg ∼ – 50 °C, Tm ∼ 60 °C) relative to the environment of living systems, excellent mechanical properties (high toughness and extensibility when molar mass is sufficiently high), and facile degradability in aqueous media where living systems function. The production of P4HB has long relied on biological fermentation, where it is stored in fermented cells and extracted at the end of the fermentation. However, the high production cost of the fermentation process, associated with its slow reaction kinetics and presently limited production volume, hinders broader implementations of P4HB. In addition, biological routes typically produce P4HB with poor control over the polymer molar mass and dispersity, and postfermentation treatment is employed to offer various molar mass P4HB formulations. Considering that chemical catalysis generally offers faster reaction kinetics, more rapid catalyst tuning, a higher degree of control, and better scalability, it would be desirable to develop a chemocatalytic route to access P4HB more rapidly, at scale, and on-demand for tailorable chain lengths and architectures. In this context, developing the effective and efficient chemocatalytic synthesis of P4HB through ring-opening polymerization (ROP) of γ-butyrolactone (γBL), which is bioderived and available at scale, is of great interest and significance.
化学合成 P4HB:从 "不可聚合 "单体到高性能生物材料的十年历程
脂肪族聚酯的每个重复单元都由可水解和/或酶降解的酯键组成,具有不同的热机械性能以及理想的生物降解性和生物相容性,因此在生物医学领域有着广泛的应用。其中,聚(4-羟基丁酸酯)(P4HB)是一种受到特别关注的生物材料,因为它具有与生物系统环境相适应的热转变温度(Tg ∼ - 50 °C,Tm ∼ 60 °C)、优异的机械性能(摩尔质量足够高时具有高韧性和延伸性)以及在生物系统起作用的水介质中的易降解性。长期以来,P4HB 的生产一直依赖于生物发酵,它储存在发酵细胞中,并在发酵结束时提取出来。然而,发酵过程的生产成本高,反应动力学慢,目前产量有限,阻碍了 P4HB 的广泛应用。此外,生物法生产的 P4HB 通常无法很好地控制聚合物摩尔质量和分散性,因此需要进行发酵后处理,以提供不同摩尔质量的 P4HB 配方。考虑到化学催化通常能提供更快的反应动力学、更迅速的催化剂调整、更高的控制程度和更好的可扩展性,我们希望能开发出一种化学催化途径,更快速、更大规模地获得 P4HB,并按需定制链长和结构。在这种情况下,开发通过γ-丁内酯(γBL)的开环聚合(ROP)来有效合成 P4HB 的化学催化途径具有极大的兴趣和意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


