Process Development for the First GMP Synthesis of SGD-9501-TFA, Part 1: Synthesis of Two Oligopeptide Fragments
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The discovery of novel auristatin-derived antibody drug conjugates (ADCs) with attenuated bystander activity is an area of intense research. Recently, drug-linker SGD-9501 emerged as a promising clinical candidate possessing favorable off-target toxicity. To support the clinical supply of ADCs based on this drug-linker, we set out to develop a first-in-human (FIH) amenable GMP manufacturing route. This report describes the process development of two oligopeptide fragments covering four synthetic steps in the seven-step convergent solution phase synthesis of SGD-9501 from commercial reagents. The highlights within this report include the discovery and development of three crystallizations, the use of PAT to control impurities formed in a direct coupling of N,N-dimethyl-O-unprotected serine, and the development of mild acid promoted boc and tert-butyl ester deprotection conditions that optimized impurity control and subsequent isolation. Each step was performed on a >500 g scale for the GMP campaign and achieved a >75% yield and >98% LC area percent (LCAP) purity.
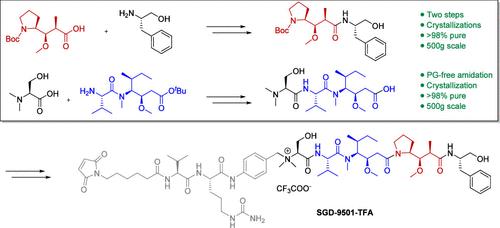
SGD-9501-TFA 首次 GMP 合成的工艺开发,第 1 部分:两个寡肽片段的合成
发现具有减弱旁观者活性的新型乌司他丁衍生抗体药物共轭物(ADC)是一个热门研究领域。最近,药物连接剂 SGD-9501 成为一种具有良好脱靶毒性的临床候选药物。为了支持以这种药物连接剂为基础的 ADC 的临床供应,我们着手开发了适合 GMP 生产工艺的首次人体 (FIH) 生产路线。本报告介绍了两个寡肽片段的工艺开发过程,涵盖了利用商业试剂七步聚合溶液相合成 SGD-9501 的四个合成步骤。本报告的亮点包括:发现和开发了三种结晶方法;使用 PAT 控制 N,N-二甲基-O-未保护丝氨酸直接偶联过程中形成的杂质;开发了弱酸促进的 boc 和叔丁酯脱保护条件,优化了杂质控制和后续分离。每个步骤都是在 500 克规模的 GMP 条件下进行的,实现了 75% 的产率和 98% 的 LC 面积百分比 (LCAP) 纯度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


