Hybrids of 3-Hydroxypyridin-4(1H)-ones and Long-Chain 4-Aminoquinolines as Potent Biofilm Inhibitors of Pseudomonas aeruginosa Potentiate Tobramycin and Polymyxin B Activity
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The biofilm formation of Pseudomonas aeruginosa involves multiple complex regulatory pathways; thus, blocking a single pathway is unlikely to achieve the desired antibiofilm efficacy. Herein, a series of hybrids of 3-hydroxypyridin-4(1H)-ones and long-chain 4-aminoquinolines were synthesized as biofilm inhibitors against P. aeruginosa based on a multipathway antibiofilm strategy. Comprehensive structure–activity relationship studies identified compound 30b as the most valuable antagonist, which significantly inhibited P. aeruginosa biofilm formation (IC50 = 5.8 μM) and various virulence phenotypes. Mechanistic studies revealed that 30b not only targets the three quorum sensing systems but also strongly induces iron deficiency signals in P. aeruginosa. Furthermore, 30b demonstrated a favorable in vitro and in vivo safety profile. Moreover, 30b specifically enhanced the antibacterial activity of tobramycin and polymyxin B in in vitro and in vivo combination therapy. Overall, these results highlight the potential of 30b as a novel anti-infective candidate for treating P. aeruginosa infections.
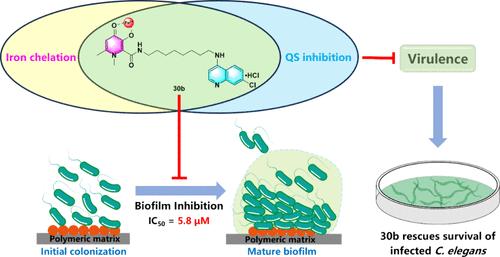
3-羟基吡啶-4(1H)-酮与长链 4-氨基喹啉的混合物作为铜绿假单胞菌的强效生物膜抑制剂,可增强妥布霉素和多粘菌素 B 的活性
铜绿假单胞菌生物膜的形成涉及多种复杂的调控途径,因此阻断单一途径不可能达到理想的抗生物膜效果。本文基于多途径抗生物膜策略,合成了一系列 3-羟基吡啶-4(1H)-酮和长链 4-氨基喹啉的混合物,作为铜绿假单胞菌的生物膜抑制剂。综合结构-活性关系研究发现化合物 30b 是最有价值的拮抗剂,它能显著抑制铜绿假单胞菌生物膜的形成(IC50 = 5.8 μM)和各种毒力表型。机理研究表明,30b 不仅针对三种法定量传感系统,还能强烈诱导铜绿假单胞菌体内的缺铁信号。此外,30b 还具有良好的体外和体内安全性。此外,在体外和体内联合疗法中,30b 还能特异性地增强妥布霉素和多粘菌素 B 的抗菌活性。总之,这些结果凸显了 30b 作为治疗铜绿假单胞菌感染的新型抗感染候选药物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


