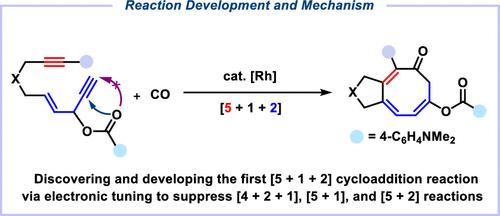
Rhodium-Catalyzed [5 + 1 + 2] Cycloaddition of Yne-3-acyloxy-1,4-enynes (YACEs) and Carbon Monoxide: Reaction Development and Mechanism
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Developing reactions to synthesize challenging eight-membered carbocycles is a research frontier of organic synthesis. Reported here is the development of Rh-catalyzed [5 + 1 + 2] cycloaddition of yne-3-acyloxy-1,4-enynes (Yne-ACEs, shortened as YACEs) and CO, in which sequentially five-carbon (generated from 3-acyloxy-1,4-enynes), one-carbon (CO), and two-carbon (alkynes) units are assembled into the final 5/8 scaffold containing a cyclooctatrienone structure. This reaction has a broad scope and can be carried out under mild conditions. Keys to the success of the present [5 + 1 + 2] reaction, discovered and supported by experiments and ab initio calculations, include using terminal alkyne in the 3-acyloxy-1,4-enyne moiety of the substrates so that 1,2-acyloxy migration (instead of 1,3-acyloxy migration, a step required for a competing [4 + 2 + 1] reaction) can be realized and applying an electron-rich aryl group (here, it is p-dimethylamino phenyl) in the acyloxy group to make a [5 + 1] pathway disfavored. Quantum chemical calculations have also been used to answer why this reaction is [5 + 1 + 2] but not [5 + 2 + 1] (where alkyne insertion is ahead of CO insertion) and to find the factors disfavoring the competitive [5 + 2], [5 + 1], and [4 + 2 + 1] reactions.
铑催化的 Yne-3-acyloxy-1,4-enynes (YACEs) 与一氧化碳的 [5 + 1 + 2] 环加成反应:反应发展与机理
开发合成具有挑战性的八元碳环的反应是有机合成的研究前沿。本文报告了在 Rh 催化下,炔-3-乙酰氧基-1,4-炔(Yne-ACEs,简称 YACEs)和 CO 的[5 + 1 + 2]环加成反应的发展情况,在该反应中,五碳(由 3-乙酰氧基-1,4-炔生成)、一碳(CO)和二碳(炔)单元依次组装成最终的 5/8 支架,其中包含环辛三烯酮结构。该反应范围广泛,可在温和的条件下进行。实验和 ab initio 计算发现并支持本[5 + 1 + 2]反应的成功关键,包括在底物的 3-acyloxy-1,4-enyne 分子中使用末端炔基,从而使 1,2-acyloxy 迁移(而不是 1、3-acyloxy 迁移,这是竞争性[4 + 2 + 1]反应所需的步骤),并在酰氧基中使用富电子芳基(此处为对二甲氨基苯基),使[5 + 1]途径不受青睐。量子化学计算也被用来回答为什么这个反应是[5 + 1 + 2]而不是[5 + 2 + 1](炔烃的插入先于 CO 的插入),并找出不利于竞争性[5 + 2]、[5 + 1]和[4 + 2 + 1]反应的因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


