Plasma membrane curvature regulates the formation of contacts with the endoplasmic reticulum
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
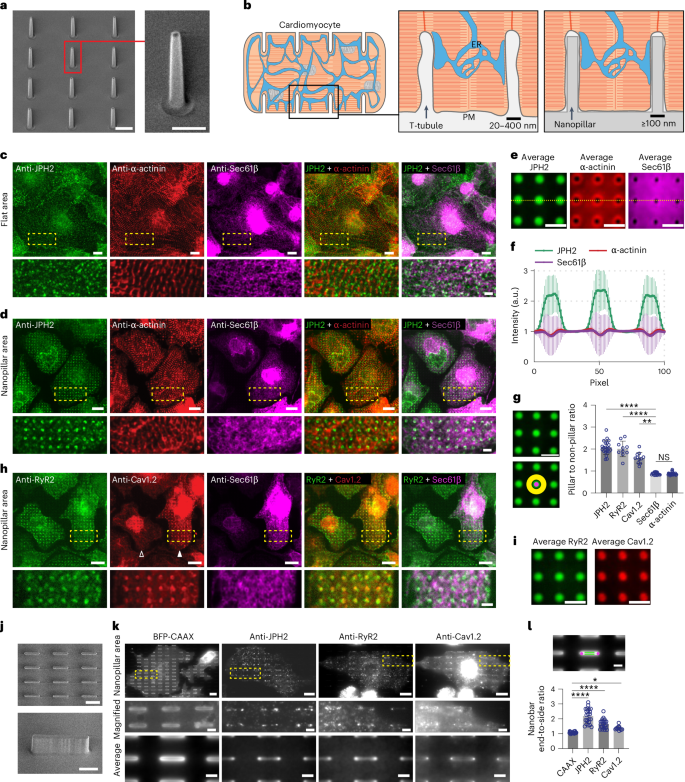
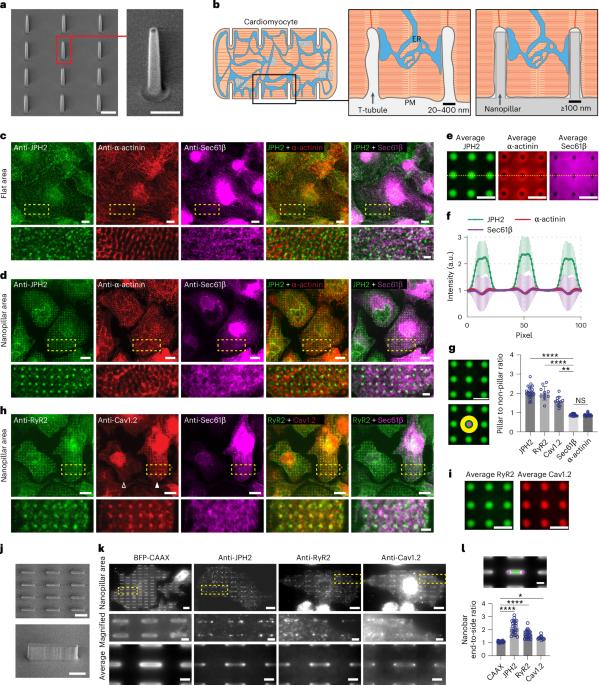
Contact sites between the endoplasmic reticulum (ER) and plasma membrane (PM) play a crucial role in governing calcium regulation and lipid homeostasis. Despite their significance, the factors regulating their spatial distribution on the PM remain elusive. Inspired by observations in cardiomyocytes, where ER–PM contact sites concentrate on tubular PM invaginations known as transverse tubules, we hypothesize that PM curvature plays a role in ER–PM contact formation. Through precise control of PM invaginations, we show that PM curvatures locally induce the formation of ER–PM contacts in cardiomyocytes. Intriguingly, the junctophilin family of ER–PM tethering proteins, specifically expressed in excitable cells, is the key player in this process, whereas the ubiquitously expressed extended synaptotagmin-2 does not show a preference for PM curvature. At the mechanistic level, we find that the low-complexity region (LCR) and membrane occupation and recognition nexus (MORN) motifs of junctophilins can bind independently to the PM, but both the LCR and MORN motifs are required for targeting PM curvatures. By examining the junctophilin interactome, we identify a family of curvature-sensing proteins—Eps15 homology domain-containing proteins—that interact with the MORN_LCR motifs and facilitate the preferential tethering of junctophilins to curved PM. These findings highlight the pivotal role of PM curvature in the formation of ER–PM contacts in cardiomyocytes and unveil a mechanism for the spatial regulation of ER–PM contacts through PM curvature modulation. Yang et al. show that plasma membrane curvature promotes the site-specific formation of contacts with the endoplasmic reticulum through junctophilin-2 tethers in cardiomyocytes.
质膜曲率调节与内质网接触的形成
内质网(ER)和质膜(PM)之间的接触点在调节钙调节和脂质平衡方面起着至关重要的作用。尽管其意义重大,但调节其在质膜上空间分布的因素仍然难以捉摸。在心肌细胞中,ER-PM 接触点集中在被称为横向小管的管状 PM 内陷处,受此观察结果的启发,我们假设 PM 的曲率在ER-PM 接触点的形成中发挥作用。通过精确控制 PM 的内陷,我们发现 PM 的曲率能在局部诱导心肌细胞中 ER-PM 接触的形成。耐人寻味的是,在兴奋细胞中特异表达的ER-PM系留蛋白的交界ophilin家族是这一过程中的关键角色,而普遍表达的扩展突触塔格明-2对PM弯曲并无偏好。在机理层面,我们发现交界嗜蛋白的低复杂度区(LCR)和膜占位与识别接头(MORN)基团可以独立地与 PM 结合,但 LCR 和 MORN 基团都是靶向 PM 弯曲所必需的。通过研究交界嗜血蛋白的相互作用组,我们发现了一个曲率感应蛋白家族--含Eps15同源结构域的蛋白--它们与MORN_LCR基序相互作用,促进交界嗜血蛋白优先拴系在弯曲的PM上。这些发现凸显了 PM 曲度在心肌细胞中形成 ER-PM 接触中的关键作用,并揭示了通过 PM 曲度调节 ER-PM 接触的空间调控机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


