Biosynthesis of peptide–nucleobase hybrids in ribosomal peptides
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
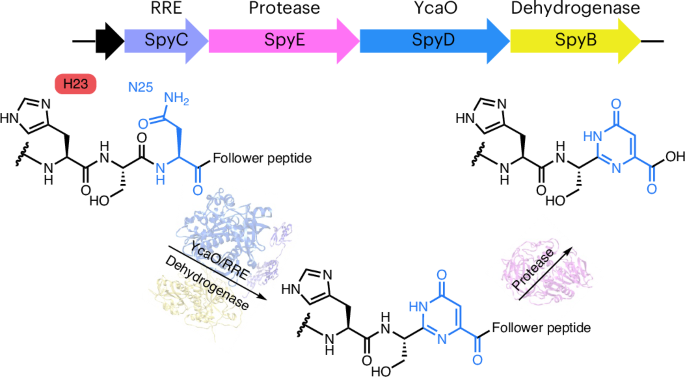
The main biopolymers in nature are oligonucleotides and polypeptides. However, naturally occurring peptide–nucleobase hybrids are rare. Here we report the characterization of the founding member of a class of peptide–nucleobase hybrid natural products with a pyrimidone motif from a widely distributed ribosomally synthesized and post-translationally modified (RiPP) biosynthetic pathway. This pathway features two steps where a heteromeric RRE–YcaO–dehydrogenase complex catalyzes the formation of a six-membered pyrimidone ring from an asparagine residue on the precursor peptide, and an acyl esterase selectively recognizes this moiety to cleave the C-terminal follower peptide. Mechanistic studies reveal that the pyrimidone formation occurs in a substrate-assisted catalysis manner, requiring a His residue in the precursor to activate asparagine for heterocyclization. Our study expands the chemotypes of RiPP natural products and the catalytic scope of YcaO enzymes. This discovery opens avenues to create artificial biohybrid molecules that resemble both peptide and nucleobase, a modality of growing interest. Naturally occurring peptide–nucleobase hybrids are rare. Here Pei et al. report the discovery and biosynthetic studies of the first peptide–nucleobase hybrid catalyzed by an RRE–YcaO–dehydrogenase complex from a RiPP pathway, and show the biotransformation in a substrate-assisted manner.

核糖体肽中肽-核碱基杂交的生物合成
自然界中的主要生物聚合物是寡核苷酸和多肽。然而,天然存在的肽-核碱基杂合物却很少见。在这里,我们报告了一类肽-核碱基杂合天然产物的创始成员的特征,该天然产物具有嘧啶酮基调,来自广泛分布的核糖体合成和翻译后修饰(RiPP)生物合成途径。这一途径有两个步骤:异构体 RRE-YcaO 脱氢酶复合物催化前体肽上的天冬酰胺残基形成六元嘧啶酮环,酰基酯酶选择性地识别这一分子,裂解 C 端从肽。机理研究显示,嘧啶酮的形成是以底物辅助催化的方式进行的,需要前体中的一个 His 残基激活天冬酰胺进行杂环化。我们的研究拓展了 RiPP 天然产物的化学类型和 YcaO 酶的催化范围。这一发现为创造既类似于肽又类似于核碱基的人工生物杂化分子开辟了道路,而这正是人们越来越感兴趣的一种模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


