Emerging Trends for the Direct Synthesis of Dithiocarbamates
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Dithiocarbamates are common structural motifs in various bioactive compounds, attracting considerable attention due to their wide‐ranging applications in organic synthesis, material science, agrochemicals, and the pharmaceutical industry. Given the significant bioactivity and extensive applications of dithiocarbamates, the continuous pursuit of their efficient and diverse synthetic methods remains critical and urgent. Direct dithiocarbamation reactions can introduce −S−C(S)NR2 group directly into parent molecules, providing shorter, greener, and more practical pathways for the synthesis of organodithiocarbamates. This article offers a comprehensive overview of the latest advancements in direct dithiocarbamation reactions, categorizing them based on the different sources of the −S−C(S)NR2 group, with a particular emphasis on elucidating substrate scope and reaction mechanisms. Additionally, some synthetic limitations and applications of these methods are discussed. This review aims to provide the latest advancements in this field to both general readers and professional practitioners, inspiring innovative ideas for the synthesis of organosulfur compounds.
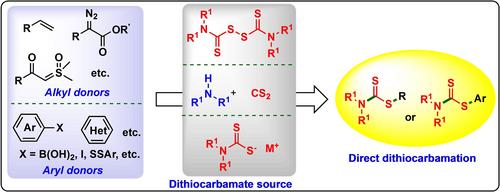
直接合成二硫代氨基甲酸盐的新趋势
二硫代氨基甲酸酯是各种生物活性化合物中常见的结构基团,因其在有机合成、材料科学、农用化学品和制药业中的广泛应用而备受关注。鉴于二硫代氨基甲酸盐具有重要的生物活性和广泛的应用,不断探索其高效和多样化的合成方法仍然是关键和紧迫的任务。直接二硫代氨基甲酸盐反应可将 -S-C(S)NR2 基团直接引入母体分子,为有机二硫代氨基甲酸盐的合成提供了更短、更环保、更实用的途径。本文全面综述了直接二硫代氨基甲酸盐反应的最新进展,并根据 -S-C(S)NR2 基团的不同来源进行了分类,尤其侧重于阐明底物范围和反应机理。此外,还讨论了这些方法的一些合成限制和应用。本综述旨在为普通读者和专业从业人员提供该领域的最新进展,启发有机硫化合物合成的创新思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


