Induced Ferroptosis Pathway by Regulating Cellular Lipid Peroxidation With Peroxynitrite Generator for Reversing “Cold” Tumors
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Overcoming the resistance of tumor cells to apoptosis and immunosuppression is an important challenge to improve tumor immunotherapy. Non-apoptotic death mode of ferroptosis has been regarded as a new strategy to enhance tumor immunotherapy against drug-resistant cancers. The lethal accumulation of lipid peroxides (LPO) determines the progress of ferroptosis. The high susceptibleness of ferroptosis provides an opportunity for combating triple-negative breast cancer. Reactive nitrogen species (RNS) produced by nitric oxide (NO) and reactive oxygen species (ROS) is more lethal than ROS for tumor cells. Herein, an RNS-mediated immunotherapy strategy for inducing ferroptosis pathway is proposed by improving LPO accumulation, and constructed a multifunctional liposome (Lipo-MT-SNAP) comprised of peroxynitrite (ONOO−) generator, tumor targeted group, inhibiting glutathione peroxidase 4 (GPX4), and basic units (dipalmitoyl phosphatidylcholine and cholesterol). The significant enhancement of LPO resulted from the intense oxidative damage of ONOO− impaired synthesis of GPX4 by depleting glutathione, which further amplified ferroptosis and triggered immunogenic cell death. In vivo, RNS-mediated photoimmunotherapy can promote polarization of M2 to M1 macrophages and dendritic cells maturation, further infiltrate T cells, regulate the secretion of inflammatory factors, and reprogram the tumor microenvironment. The powerful RNS-mediated ferroptosis induces strong immunogenicity and effectively inhibit tumor proliferation.
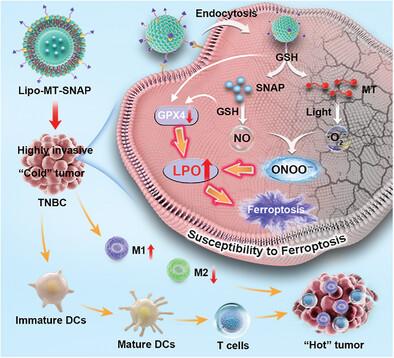
利用过氧化亚硝酸盐发生器调节细胞脂质过氧化,诱导铁氧化途径,从而逆转 "冷 "肿瘤
克服肿瘤细胞对凋亡和免疫抑制的抵抗是提高肿瘤免疫疗法的一个重要挑战。铁凋亡的非凋亡死亡模式已被视为一种新策略,可增强针对耐药性癌症的肿瘤免疫疗法。脂质过氧化物(LPO)的致命积累决定了铁凋亡的进程。铁变态反应的高度易感性为抗击三阴性乳腺癌提供了机会。一氧化氮(NO)和活性氧(ROS)产生的活性氮物(RNS)比 ROS 对肿瘤细胞的杀伤力更大。本文提出了一种以 RNS 为介导的免疫治疗策略,通过改善 LPO 的积累来诱导铁变态反应途径,并构建了一种多功能脂质体(Lipo-MT-SNAP),由过亚硝酸盐(ONOO-)发生器、肿瘤靶向基团、抑制谷胱甘肽过氧化物酶 4(GPX4)和基本单元(二棕榈酰基磷脂酰胆碱和胆固醇)组成。ONOO 的强烈氧化损伤通过消耗谷胱甘肽而损害了 GPX4 的合成,从而导致 LPO 明显增强,这进一步扩大了铁变态反应并引发免疫性细胞死亡。在体内,RNS 介导的光免疫疗法可促进 M2 巨噬细胞极化为 M1 巨噬细胞和树突状细胞成熟,进一步浸润 T 细胞,调节炎症因子的分泌,并对肿瘤微环境进行重编程。强大的 RNS 介导的铁跃迁诱导了强大的免疫原性,有效抑制了肿瘤增殖。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


