Photochemical CO2 hydrogenation to carbon nanotubes and H2O for oxygen recovery in space exploration
IF 38.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The primary source of oxygen in space exploration is derived from water electrolysis. Herein, we discovered a mild photochemical hydrogenation process that can convert CO2 into carbon nanotubes (CNTs) and H2O by using a Co-based catalyst. Hence, astronauts can extract oxygen from CO2 metabolism to close the oxygen recycling loop (overall reaction: CO2 → C + O2), allowing for ∼100% theoretical oxygen recovery. This photochemical technique has enabled a high turnover number (the molar ratio of C to Co) of 240 for CNT formation during a 100 h reaction in a flow reactor. The oxygen recovery efficiency reaches approximately 68% when using flowing CO2 and H2, surpassing the theoretical maximum (50%) for the Sabatier reaction combined with water electrolysis at the International Space Station. The tip-growth mode of CNTs principally allows long-term oxygen recovery from CO2, in addition to space manufacturing of CNTs.
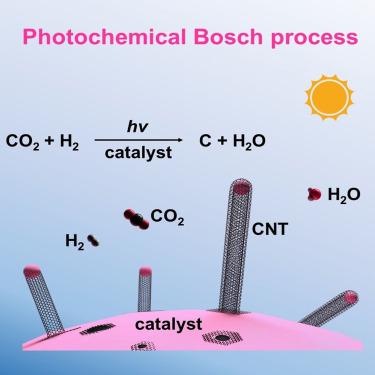
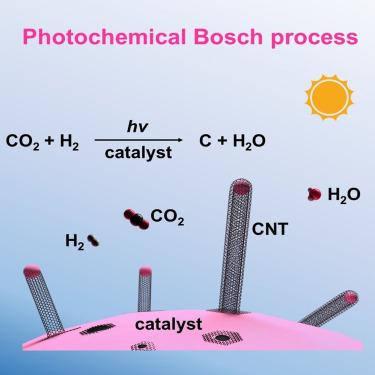
光化学二氧化碳加氢生成碳纳米管和 H2O,用于太空探索中的氧气回收
太空探索中氧气的主要来源是电解水。在此,我们发现了一种温和的光化学氢化过程,通过使用 Co 基催化剂,可将 CO2 转化为碳纳米管(CNTs)和 H2O。因此,宇航员可以从二氧化碳代谢中提取氧气,从而完成氧气回收循环(总反应:CO2 → C + O2),使理论氧气回收率达到 100%。在流动反应器中进行 100 小时的反应期间,这种光化学技术使 CNT 的形成达到 240 的高周转数(Co 与 Co 的摩尔比)。当使用流动的二氧化碳和 H2 时,氧气回收效率达到约 68%,超过了国际空间站结合水电解进行的萨巴蒂尔反应的理论最大值(50%)。除了在太空中制造碳纳米管外,碳纳米管的尖端生长模式主要允许从二氧化碳中长期回收氧气。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
文献相关原料
公司名称
产品信息
阿拉丁
cobalt powder
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


