TREM2 alleviates long-term cognitive dysfunction after subarachnoid hemorrhage in mice by attenuating hippocampal neuroinflammation via PI3K/Akt signaling pathway
Abstract
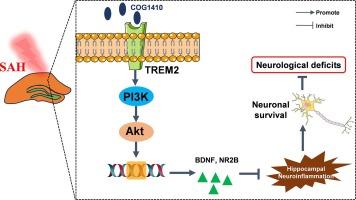
Subarachnoid hemorrhage (SAH) often leads to long-term cognitive deficits in patients, particularly due to injury to brain regions such as the hippocampus. This study aims to investigate the role of the triggering receptor expressed on myeloid cells 2 (TREM2) in mitigating hippocampal injury and associated cognitive impairments following SAH. To explore the protective effects of TREM2, we utilized the TREM2 agonist COG1410 to upregulate TREM2 expression and employed TREM2 knockout (KO) mice to verify the necessity of TREM2 for this protective role. The study further examined the involvement of the PI3K/Akt signaling pathway in TREM2-mediated neuroprotection. Our findings indicate that the upregulation of TREM2 significantly alleviated long-term cognitive deficits and promoted the recovery of hippocampal neural activity post-SAH. The neuroprotective effects were linked to reduced microglial activation and decreased secretion of inflammatory factors within the hippocampus. In contrast, TREM2 KO mice did not exhibit these protective effects. Furthermore, inhibition of the PI3K/Akt pathway also diminished these protective effects of TREM2 upregulation and worsened cognitive outcomes. In conclusion, TREM2 upregulation mitigates long-term cognitive dysfunction following SAH by attenuating hippocampal neuroinflammation via the PI3K/Akt signaling pathway. These findings suggest that TREM2 could be a potential therapeutic target for improving cognitive outcomes after SAH.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


