Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
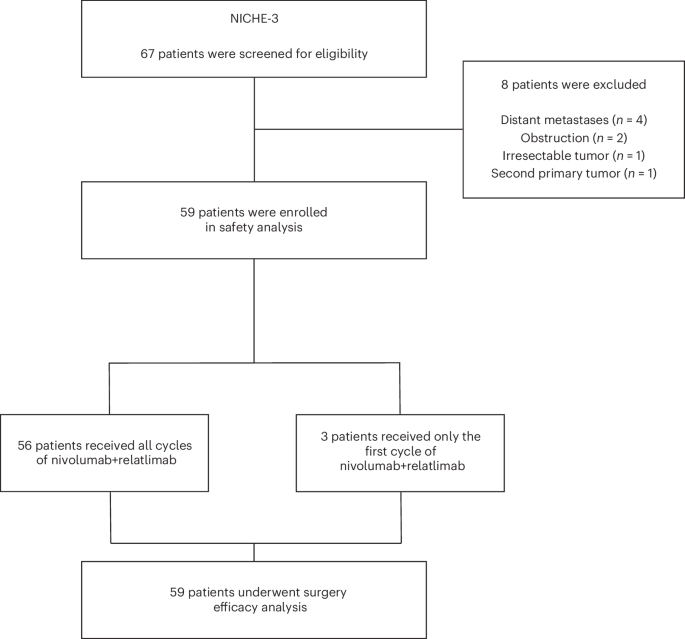
Mismatch repair deficiency (dMMR) is found in approximately 15% of non-metastatic colon cancers (CCs) and is characterized by a defective DNA mismatch repair system, resulting in hypermutated and highly immunogenic tumors. Although patients with dMMR CC have limited benefit from chemotherapy, these tumors have been shown to respond exceptionally well to neoadjuvant anti-PD-1 plus anti-CTLA-4, with high rates of pathologic responses. Here, based on data from melanoma studies, we postulated a high efficacy and favorable toxicity profile of anti-PD-1 plus anti-LAG-3. In the NICHE-3 study, a total of 59 patients with locally advanced dMMR CC were treated with two 4-weekly cycles of nivolumab (480 mg) plus relatlimab (480 mg) before surgery. Pathologic response was observed in 57 of 59 (97%; 95% confidence interval (CI): 88–100%) patients, meeting the primary endpoint. Responses included 54 (92%; 95% CI: 81–97%) major pathologic responses (≤10% residual viable tumor) and 40 (68%; 95% CI: 54–79%) pathologic complete responses. With a median follow-up of 8 months (range, 2–19), one patient had recurrence of disease. The treatment displayed an acceptable safety profile, with all-grade and grade 3–4 immune-related adverse events (irAEs) occurring in 80% and 10% of patients, respectively. The most common irAEs were infusion-related reactions (29%), thyroid dysfunction (22%) and fatigue (20%). In conclusion, our results show that neoadjuvant nivolumab/relatlimab induces high rates of pathologic responses and that further investigation of this treatment in larger studies is warranted. These data add to the body of evidence in support of neoadjuvant immunotherapy regimens in dMMR CC. ClinicalTrials.gov identifier: NCT03026140 . In the phase 2 NICHE-3 trial, patients with locally advanced mismatch repair-deficient colon cancer who were treated with neoadjuvant anti-PD1 and anti-LAG3 agents showed high rates of pathological responses, requiring validation in larger trials.
局部晚期MMR缺陷结肠癌新辅助nivolumab和relatlimab治疗:2期试验
大约15%的非转移性结肠癌(CC)中存在错配修复缺陷(dMMR),其特点是DNA错配修复系统缺陷,导致肿瘤高突变和高免疫原性。尽管dMMR CC患者从化疗中获益有限,但这些肿瘤已被证明对新辅助抗PD-1加抗CTLA-4反应特别好,病理反应率很高。在此,根据黑色素瘤研究的数据,我们推测抗PD-1加抗LAG-3具有较高的疗效和良好的毒性。在NICHE-3研究中,共有59名局部晚期dMMR CC患者在手术前接受了两个4周周期的nivolumab(480毫克)加relatlimab(480毫克)治疗。59例患者中有57例(97%;95%置信区间(CI):88-100%)出现了病理应答,达到了主要终点。反应包括54例(92%;95% CI:81-97%)主要病理反应(残留存活肿瘤≤10%)和40例(68%;95% CI:54-79%)病理完全反应。中位随访时间为 8 个月(2-19 个月),其中一名患者疾病复发。该疗法的安全性尚可,80%和10%的患者分别出现了所有等级和3-4级免疫相关不良事件(irAEs)。最常见的不良反应是输液相关反应(29%)、甲状腺功能障碍(22%)和疲劳(20%)。总之,我们的研究结果表明,新辅助nivolumab/relatlimab能诱导较高比例的病理反应,因此有必要在更大规模的研究中进一步探讨这种治疗方法。这些数据为新辅助免疫疗法治疗dMMR CC提供了更多证据。ClinicalTrials.gov 标识符:NCT03026140:NCT03026140。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


