Tetrahedral Framework Nucleic Acids Delivery of Pirfenidone for Anti-Inflammatory and Antioxidative Effects to Treat Idiopathic Pulmonary Fibrosis
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
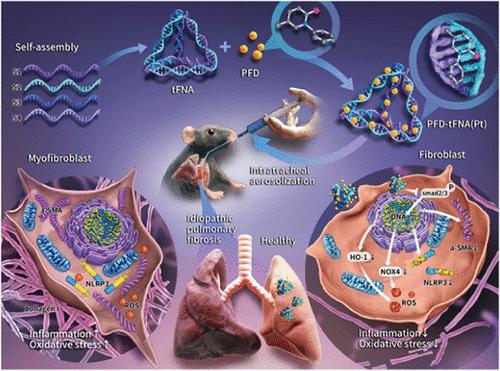
Idiopathic pulmonary fibrosis (IPF) is a chronic and irreversible lung disease, and developing an effective treatment remains a challenge. The limited therapeutic options are primarily delivered by the oral route, among which pirfenidone (PFD) improves pulmonary dysfunction and patient quality of life. However, its high dose and severe side effects (dyspepsia and systemic photosensitivity) limit its clinical value. Intratracheal aerosolization is an excellent alternative method for treating lung diseases because it increases the concentration of the drug needed to reach the focal site. Tetrahedral framework nucleic acid (tFNA) is a drug delivery system with exceptional delivery capabilities. Therefore, we synthesized a PFD-tFNA (Pt) complex using tFNA as the delivery vehicle and achieved quantitative nebulized drug delivery to the lungs via micronebulizer for lung fibrosis treatment. In vivo, Pt exhibited excellent immunomodulatory capacity and antioxidant effects. Furthermore, Pt reduced mortality, gradually restored body weight and improved lung tissue structure. Similarly, Pt also exhibited superior fibrosis inhibition in an in vitro fibrosis model, as shown by the suppression of excessive fibroblast activation and epithelial-mesenchymal transition (EMT) in epithelial cells exposed to TGF-β1. Conclusively, Pt, a complex with tFNA as a transport system, could enrich the therapeutic regimen for IPF via intratracheal aerosolization inhalation.
四面体框架核酸递送吡非尼酮的抗炎和抗氧化作用治疗特发性肺纤维化
特发性肺纤维化(IPF)是一种不可逆的慢性肺部疾病,开发有效的治疗方法仍是一项挑战。有限的治疗方案主要通过口服途径给药,其中吡非尼酮(PFD)可改善肺功能障碍和患者的生活质量。然而,高剂量和严重的副作用(消化不良和全身光敏性)限制了其临床价值。气管内雾化是治疗肺部疾病的最佳替代方法,因为它能提高到达病灶部位所需的药物浓度。四面体框架核酸(tFNA)是一种具有卓越给药能力的给药系统。因此,我们以 tFNA 为给药载体合成了 PFD-tFNA (Pt) 复合物,并通过微雾化器实现了定量雾化给药到肺部,用于治疗肺纤维化。在体内,铂具有出色的免疫调节能力和抗氧化作用。此外,铂还能降低死亡率、逐渐恢复体重并改善肺组织结构。同样,在体外纤维化模型中,铂也表现出卓越的纤维化抑制能力,这体现在它能抑制暴露于 TGF-β1 的上皮细胞中成纤维细胞的过度活化和上皮-间质转化(EMT)。总之,以 tFNA 为转运系统的铂复合物可以通过气管内雾化吸入丰富 IPF 的治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


