Carrier-Free Nanoagent Interfering with Cancer-Associated Fibroblasts’ Metabolism to Promote Tumor Penetration for Boosted Chemotherapy
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Elevated production of extracellular matrix (ECM) in tumor stroma is a critical obstacle for drug penetration. Here we demonstrate that ATP-citrate lyase (ACLY) is significantly upregulated in cancer-associated fibroblasts (CAFs) to produce tumor ECM. Using a self-assembling nanoparticle-design approach, a carrier-free nanoagent (CFNA) is fabricated by simply assembling NDI-091143, a specific ACLY inhibitor, and doxorubicin (DOX) or paclitaxel (PTX), the first-line chemotherapeutic drug, via multiple noncovalent interactions. After arriving at the CAFs-rich tumor site, NDI-091143-mediated ACLY inhibition in CAFs can block the de novo synthesis of fatty acid, thereby dampening the fatty acid-involved energy metabolic process. As the lack of enough energy, the energetic CAFs will be in a dispirited state that is unable to produce abundant ECM, thereby significantly improving drug perfusion in tumors and enhancing the efficacy of chemotherapy. Such a simple drug assembling strategy aimed at CAFs’ ACLY-mediated metabolism pathway presents the feasibility of stromal matrix reduction to potentiate chemotherapy.
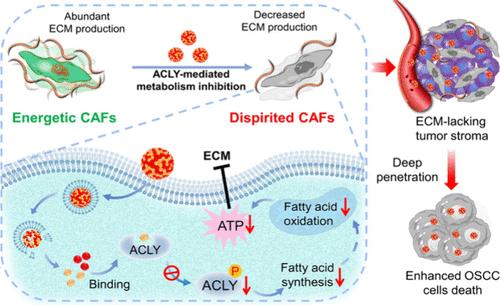
无载体纳米试剂干扰癌症相关成纤维细胞的新陈代谢,促进肿瘤穿透以增强化疗效果
肿瘤基质中细胞外基质(ECM)的生成增加是药物渗透的关键障碍。在这里,我们证明了ATP-柠檬酸裂解酶(ACLY)在癌症相关成纤维细胞(CAFs)中显著上调,从而产生肿瘤ECM。我们采用自组装纳米粒子设计方法,将特异性 ACLY 抑制剂 NDI-091143 和一线化疗药物多柔比星(DOX)或紫杉醇(PTX)通过多种非共价相互作用组装在一起,制成了一种无载体纳米试剂(CFNA)。NDI-091143介导的ACLY抑制剂到达富含CAFs的肿瘤部位后,可阻断CAFs中脂肪酸的从头合成,从而抑制脂肪酸参与的能量代谢过程。由于缺乏足够的能量,精力充沛的CAFs将处于萎靡状态,无法产生丰富的ECM,从而显著改善肿瘤内的药物灌注,提高化疗的疗效。这种针对 CAFs ACLY 介导的新陈代谢途径的简单药物组装策略,展示了基质减少以增强化疗效果的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


