Rapid and Highly Selective Fe(IV) Generation by Fe(II)-Peroxyacid Advanced Oxidation Processes: Mechanistic Investigation via Kinetics and Density Functional Theory
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
High-valent iron (Fe(IV/V/VI)) has been widely applied in water decontamination. However, common Fe(II)-activating oxidants including hydrogen peroxide (H2O2) and persulfate react slowly with Fe(II) and exhibit low selectivity for Fe(IV) production due to the cogeneration of radicals. Herein, we report peroxyacids (POAs; R–C(O)OOH) that can react with Fe(II) more than 3 orders of magnitude faster than H2O2, with high selectivity for Fe(IV) generation. Rapid degradation of bisphenol A (BPA, an endocrine disruptor) was achieved by the combination of Fe(II) with performic acid (PFA), peracetic acid (PAA), or perpropionic acid (PPA) within one second. Experiments with phenyl methyl sulfoxide (PMSO) and tert-butyl alcohol (TBA) revealed Fe(IV) as the major reactive species in all three Fe(II)-POA systems, with a minor contribution of radicals (i.e., •OH and R–C(O)O•). To understand the exceptionally high reactivity of POAs, a detailed computational comparison among the Fenton-like reactions with step-by-step thermodynamic evaluation was conducted. The high reactivity is attributed to the lower energy barriers for O–O bond cleavage, which is determined as the rate-limiting step for the Fenton-like reactions, and the thermodynamically favorable bidentate binding pathway of POA with iron. Overall, this study advances knowledge on POAs as novel Fenton-like reagents and sheds light on computational chemistry for these systems.
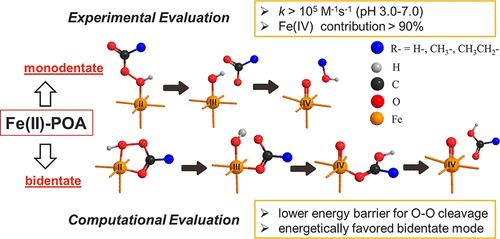
通过 Fe(II)-Peroxyacid 高级氧化过程快速、高选择性生成 Fe(IV):通过动力学和密度泛函理论进行机理研究
高价铁(Fe(IV/V/VI))已被广泛应用于水净化领域。然而,过氧化氢(H2O2)和过硫酸盐等常见的铁(II)活化氧化剂与铁(II)的反应速度较慢,并且由于自由基的共生,铁(IV)生成的选择性较低。在此,我们报告了过氧酸(POAs;R-C(O)OOH),它与铁(II)的反应速度比 H2O2 快 3 个数量级以上,对铁(IV)的生成具有高选择性。Fe(II) 与执行酸 (PFA)、过乙酸 (PAA) 或过丙酸 (PPA) 的结合可在一秒钟内实现双酚 A(BPA,一种内分泌干扰物)的快速降解。用苯基甲基亚砜(PMSO)和叔丁醇(TBA)进行的实验显示,Fe(IV) 是所有三种 Fe(II)-POA 系统中的主要反应物,自由基(即 -OH 和 R-C(O)O-)的贡献较小。为了了解 POAs 的超高反应活性,我们对 Fenton 类反应进行了详细的计算比较,并逐步进行了热力学评估。高反应性归因于 O-O 键裂解的较低能垒(被确定为 Fenton 类反应的限速步骤),以及 POA 与铁的热力学有利双叉结合途径。总之,这项研究增进了人们对作为新型芬顿类试剂的 POA 的了解,并为这些系统的计算化学提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


