Datopotamab–deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
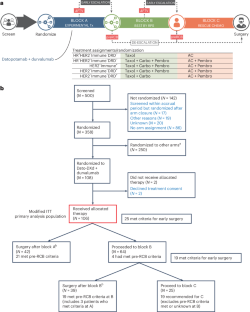
Sequential adaptive trial designs can help accomplish the goals of personalized medicine, optimizing outcomes and avoiding unnecessary toxicity. Here we describe the results of incorporating a promising antibody–drug conjugate, datopotamab–deruxtecan (Dato-DXd) in combination with programmed cell death-ligand 1 inhibitor, durvalumab, as the first sequence of therapy in the I-SPY2.2 phase 2 neoadjuvant sequential multiple assignment randomization trial for high-risk stage 2/3 breast cancer. The trial includes three blocks of treatment, with initial randomization to different experimental agent(s) (block A), followed by a taxane-based regimen tailored to tumor subtype (block B), followed by doxorubicin–cyclophosphamide (block C). Subtype-specific algorithms based on magnetic resonance imaging volume change and core biopsy guide treatment redirection after each block, including the option of early surgical resection in patients predicted to have a high likelihood of pathologic complete response, which is the primary endpoint assessed when resection occurs. There are two primary efficacy analyses: after block A and across all blocks for six prespecified HER2-negative subtypes (defined by hormone receptor status and/or response-predictive subtypes). In total, 106 patients were treated with Dato-DXd/durvalumab in block A. In the immune-positive subtype, Dato-DXd/durvalumab exceeded the prespecified threshold for success (graduated) after block A; and across all blocks, pathologic complete response rates were equivalent to the rate expected for the standard of care (79%), but 54% achieved that result after Dato-DXd/durvalumab alone (block A) and 92% without doxorubicin–cyclophosphamide (after blocks A + B). The treatment strategy across all blocks graduated in the hormone-negative/immune-negative subtype. No new toxicities were observed. Stomatitis was the most common side effect in block A. No patients receiving block A treatment alone had adrenal insufficiency. Dato-DXd/durvalumab is a promising therapy combination that can eliminate standard chemotherapy in many patients, particularly the immune-positive subtype. ClinicalTrials.gov registration: NCT01042379 . In the I-SPY2.2 trial, patients with high-risk stage 2/3 breast cancer received neoadjuvant datopotamab–deruxtecan plus durvalumab, followed by sequential chemotherapy with or without targeted therapy, with the option of early surgical resection after each block of therapy, showing that de-escalation of therapy is possible for several patient subgroups without compromising outcome and avoiding toxicity of standard chemotherapy.

达托帕单抗-德鲁司坦加杜瓦单抗治疗早期乳腺癌:顺序多重分配随机I-SPY2.2第二阶段试验
序贯适应性试验设计有助于实现个性化医疗的目标,优化疗效并避免不必要的毒性。在此,我们介绍了在高风险2/3期乳腺癌I-SPY2.2 2期新辅助序贯多重赋值随机试验中,将有前景的抗体药物共轭物达托帕单抗-德鲁司坦(Dato-DXd)与程序性细胞死亡配体1抑制剂杜瓦鲁单抗(durvalumab)联合作为第一序列治疗的结果。该试验包括三个治疗区块,首先随机分配不同的试验药物(A 区块),然后是根据肿瘤亚型定制的以紫杉类药物为基础的治疗方案(B 区块),最后是多柔比星-环磷酰胺(C 区块)。基于磁共振成像体积变化和核心活检的亚型特异性算法指导每个区段后的治疗调整,包括对预测病理完全反应可能性高的患者选择早期手术切除,这是在切除时评估的主要终点。主要疗效分析有两项:A区治疗后的疗效分析和所有区治疗后的疗效分析,分别针对六个预先指定的HER2阴性亚型(由激素受体状态和/或反应预测亚型定义)。在免疫阳性亚型中,A区后Dato-DXd/durvalumab超过了预设的成功阈值(分级);在所有区中,病理完全应答率与标准治疗的预期率(79%)相当,但单用Dato-DXd/durvalumab后(A区)有54%的患者达到了这一结果,在不使用多柔比星-环磷酰胺的情况下(A+B区后)有92%的患者达到了这一结果。在激素阴性/免疫阴性亚型中,所有区段的治疗策略均已毕业。未观察到新的毒性反应。口腔炎是A区最常见的副作用,没有单独接受A区治疗的患者出现肾上腺功能不全。Dato-DXd/durvalumab是一种很有前景的治疗组合,可以消除许多患者的标准化疗,尤其是免疫阳性亚型患者:NCT01042379。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


