Process Development for the First GMP Synthesis of SGD-9501-TFA, Part 2: Synthesis of the Payload, Linker, and Drug Linker
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The discovery of novel auristatin-derived antibody drug conjugates (ADCs) with attenuated bystander activity is an area of intense research. Recently, drug-linker SGD-9501-TFA emerged as a promising clinical candidate possessing a favorable off-target toxicity profile. To support the clinical development of ADCs utilizing this drug linker, we set out to develop a first-in-human amenable Good Manufacturing Practice manufacturing route. In this report, we describe the discovery and development of three of seven synthetic steps in convergent solution-phase synthesis. The activation of the linker is achieved with SOCl2 in NMP (step 6), and solutions to the challenges associated with isolation and stability are described. Next, novel HTE platforms used to explore peptide coupling and crystallization for the synthesis of the payload, auristatin S, are unveiled (step 5). Finally, the synthesis of the quaternary ammonium drug linker, SGD-9501-TFA, with a NaI-mediated benzylic amination, is described (step 7). We also discuss solutions to a eutectic gelation risk to direct precipitation of the crude drug linker and a trifluoroacetate ester impurity forming during lyophilization.
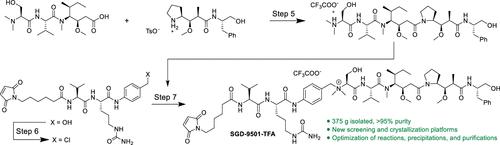
SGD-9501-TFA 首次 GMP 合成的工艺开发,第 2 部分:有效载荷、连接体和药物连接体的合成
发现具有减弱旁观者活性的新型乌司他丁衍生抗体药物共轭物(ADC)是一个热门研究领域。最近,药物连接剂 SGD-9501-TFA 成为了一种具有良好脱靶毒性的临床候选药物。为了支持使用这种药物连接剂的 ADC 的临床开发,我们着手开发了第一条适用于人体的良好生产规范生产路线。在本报告中,我们介绍了聚合溶液相合成法七个合成步骤中三个步骤的发现和开发过程。在 NMP 中使用 SOCl2 实现了连接体的活化(步骤 6),并介绍了与分离和稳定性相关的难题的解决方案。接下来,揭示了用于探索肽偶联和结晶合成有效载荷 auristatin S 的新型 HTE 平台(步骤 5)。最后,介绍了通过 NaI 介导的苄基氨基化合成季铵药物连接体 SGD-9501-TFA(步骤 7)。我们还讨论了解决共晶凝胶化风险、直接沉淀粗制药物连接剂和冻干过程中形成的三氟乙酸酯杂质的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


