Inhalable SPRAY nanoparticles by modular peptide assemblies reverse alveolar inflammation in lethal Gram-negative bacteria infection
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
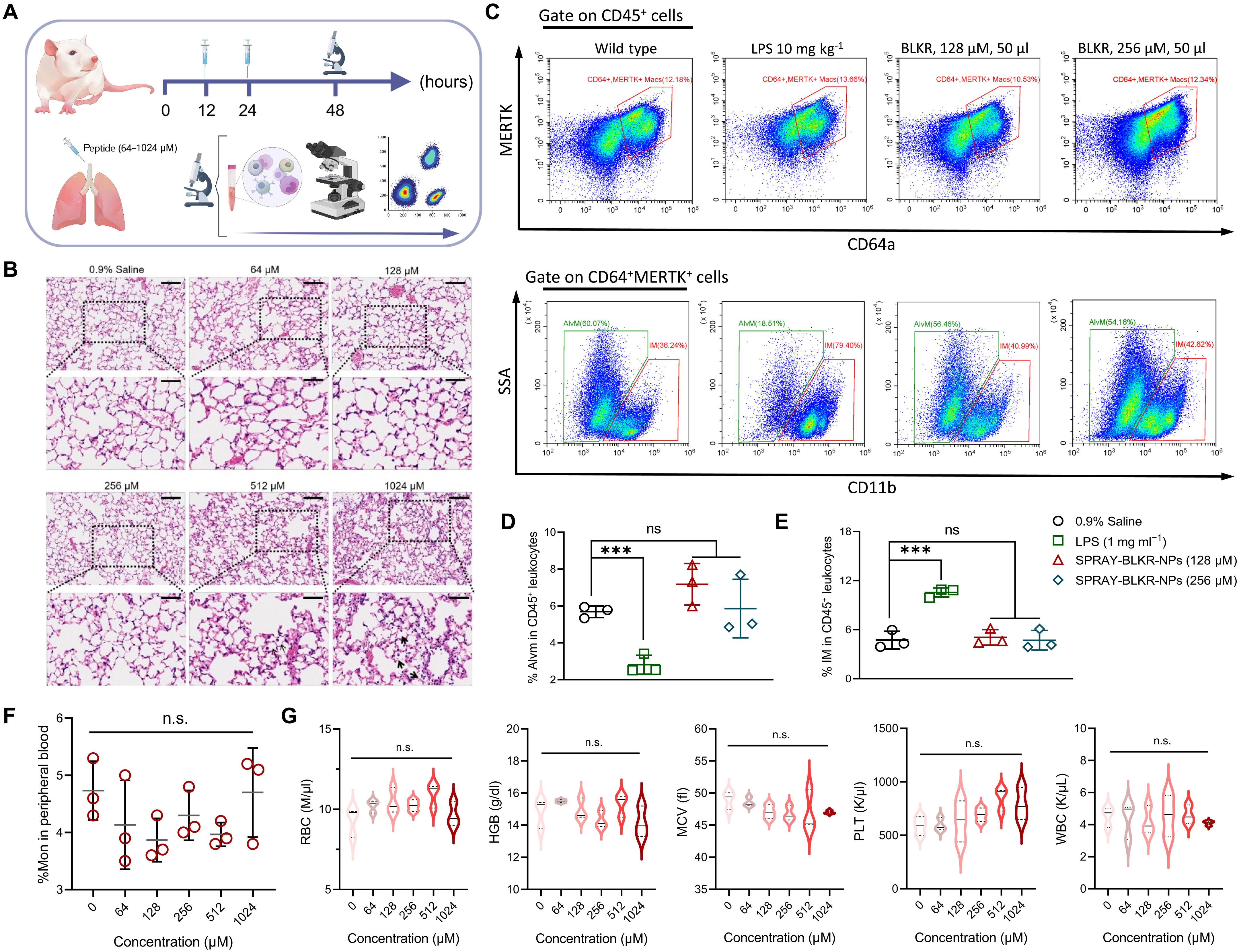
Current pharmacotherapy remains futile in acute alveolar inflammation induced by Gram-negative bacteria (GNB), eliciting consequent respiratory failure. The release of lipid polysaccharides after antibiotic treatment and subsequent progress of proinflammatory cascade highlights the necessity to apply effective inflammation management simultaneously. This work describes modular self-assembling peptides for rapid anti-inflammatory programming (SPRAY) to form nanoparticles targeting macrophage specifically, having anti-inflammation and bactericidal functions synchronously. SPRAY nanoparticles accelerate the self-delivery process in macrophages via lysosomal membrane permeabilization, maintaining anti-inflammatory programming in macrophages with efficacy close to T helper 2 cytokines. By pulmonary deposition, SPRAY nanoparticles effectively suppress inflammatory infiltration and promote alveoli regeneration in murine aseptic acute lung injury. Moreover, SPRAY nanoparticles efficiently eradicate multidrug-resistant GNB in alveoli by disrupting bacterial membrane. The universal molecular design of SPRAY nanoparticles provides a robust and clinically unseen local strategy in reverse acute inflammation featured by a high accumulation of proinflammatory cellularity and drug-resistant bacteria.
模块化多肽组装的可吸入 SPRAY 纳米粒子可逆转致命革兰氏阴性菌感染中的肺泡炎症
革兰氏阴性菌(GNB)诱发的急性肺泡炎症会导致呼吸衰竭,而目前的药物治疗仍然徒劳无益。抗生素治疗后脂质多糖的释放以及随后促炎级联反应的进展,突出表明了同时应用有效炎症管理的必要性。这项研究介绍了用于快速抗炎编程的模块化自组装肽(SPRAY),它能形成专门针对巨噬细胞的纳米颗粒,同时具有抗炎和杀菌功能。SPRAY 纳米粒子通过溶酶体膜渗透加速巨噬细胞的自我递送过程,维持巨噬细胞的抗炎程序,其功效接近 T 辅助 2 细胞因子。通过肺沉积,SPRAY 纳米粒子能有效抑制炎症浸润,促进小鼠无菌性急性肺损伤的肺泡再生。此外,SPRAY 纳米粒子还能通过破坏细菌膜有效清除肺泡中的耐多药 GNB。SPRAY 纳米粒子的通用分子设计为逆转以促炎症细胞和耐药细菌高度聚集为特征的急性炎症提供了一种稳健且临床上未见的局部策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


