Transcranial direct current stimulation enhances the protective effect of isoflurane preconditioning on cerebral ischemia/reperfusion injury: A new mechanism associated with the nuclear protein Akirin2
Abstract
Aims
Ischemic stroke is a major cause of disability and mortality worldwide. Transcranial direct current stimulation (tDCS) and isoflurane (ISO) preconditioning exhibit neuroprotective properties. However, it remains unclear whether tDCS enhances the protective effect of ISO preconditioning on ischemic stroke, and the underlying mechanisms are yet to be clarified.
Method
A model of middle cerebral artery occlusion (MCAO), a rat ischemia–reperfusion (I/R) injury model, and an in vitro oxygen–glucose deprivation/re-oxygenation (O/R) model of ischemic injury were developed. ISO preconditioning and tDCS were administered daily for 7 days before MCAO modeling. Triphenyltetrazolium chloride staining, modified neurological severity score, and hanging-wire test were conducted to assess infarct volume and neurological outcomes. Untargeted metabolomic experiments, adeno-associated virus, lentiviral vectors, and small interfering RNA techniques were used to explore the underlying mechanisms.
Results
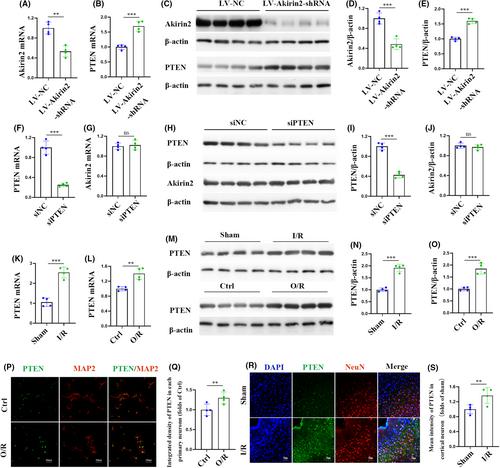
tDCS/DCS enhanced the protective effects of ISO pretreatment on I/R injury-induced brain damage. This was evidenced by reduced infarct volume and improved neurological outcomes in rats with MCAO, as well as decreased cortical neuronal death after O/R injury. Untargeted metabolomic experiments identified oxidative phosphorylation (OXPHOS) as a critical pathological process for ISO-mediated neuroprotection from I/R injury. The combination of tDCS/DCS with ISO preconditioning significantly inhibited I/R injury-induced OXPHOS. Mechanistically, Akirin2, a small nuclear protein that regulates cell proliferation and differentiation, was found to decrease in the cortex of rats with MCAO and in cortical primary neurons subjected to O/R injury. Akirin2 functions upstream of phosphatase and tensin homolog deleted on chromosome 10 (PTEN). tDCS/DCS was able to further upregulate Akirin2 levels and activate the Akirin2/PTEN signaling pathway in vivo and in vitro, compared with ISO pretreatment alone, thereby contributing to the improvement of cerebral I/R injury.
Conclusion
tDCS treatment enhances the neuroprotective effects of ISO preconditioning on ischemic stroke by inhibiting oxidative stress and activating Akirin2-PTEN signaling pathway, highlighting potential of combination therapy in ischemic stroke.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


