Synthesis, characterization, theoretical, and experimental evaluation of novel imidazolone − based compounds as eco-friendly corrosion inhibitors for mild steel
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2024-08-26
DOI:10.1016/j.jiec.2024.08.042
引用次数: 0
Abstract
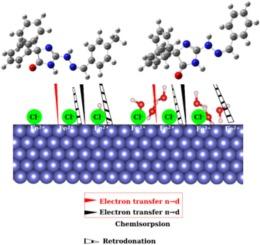
This study investigates the corrosion inhibition properties of two newly synthesized namely (E)-2-(2-benzylidenehydrazineyl)-5,5-diphenyl-3,5-dihydro-4H-imidazol-4-one denoted Ph-DDI and (E)-2-(2-(4-methylbenzylidene)hydrazineyl)-5,5-diphenyl-3,5-dihydro-4H-imidazol-4-one denoted CH3Ph-DDI, on mild steel (MS) in a 1 M HCl solution. These compounds demonstrated high inhibition efficiencies of 98.3 % and 98.7 %, respectively. Structural characterization was performed using FT-IR, 1H NMR, 13C NMR, and HRMS-ESI. Theoretical evaluations indicated high reactivity and potent inhibition capacity. Electrochemical tests confirmed a concentration-dependent inhibition effectiveness up to 328 K. Adsorption studies suggested that the compounds displace water molecules to form an adsorbed protective layer. Microscopy analysis provided insights into the corrosion inhibition mechanisms, confirming the formation of protective layers and iron/inhibitor complexes. Further molecular structure analysis using Monte Carlo (MC) simulations and density functional theory (DFT) calculations elucidated the structural features contributing to the compounds’ effective corrosion inhibition properties.
作为低碳钢环保型缓蚀剂的新型咪唑啉酮化合物的合成、表征、理论和实验评估
本研究探讨了两种新合成物,即(E)-2-(2-亚苄基肼基)-5,5-二苯基-3,5-二氢咪唑-4-酮和(E)-2-(2-(4-甲基亚苄基)肼基)-5,5-二苯基-3,5-二氢咪唑-4-酮在 1 M HCl 溶液中对低碳钢 (MS) 的缓蚀性能。这些化合物的抑制率分别高达 98.3% 和 98.7%。利用 FT-IR、H NMR、C NMR 和 HRMS-ESI 进行了结构表征。理论评估表明,该化合物具有很高的反应活性和很强的抑制能力。吸附研究表明,这些化合物取代了水分子,形成了吸附保护层。显微镜分析深入揭示了腐蚀抑制机制,证实了保护层和铁/抑制剂复合物的形成。利用蒙特卡罗(MC)模拟和密度泛函理论(DFT)计算进行的进一步分子结构分析阐明了化合物有效缓蚀特性的结构特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


