ROS-Responsive and Self-Catalytic Nanocarriers for a Combination of Chemotherapy and Reinforced Ferroptosis against Breast Cancer
IF 5.4
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Ferroptosis is an appealing cancer therapy strategy based on the H2O2-involved Fenton reaction to produce toxic •OH for lipid peroxidation. However, intracellular H2O2 is easily consumed and results in a deficient Fenton reaction. This obstacle can be overcome by traditional chemotherapeutic drugs for H2O2 supplements. Moreover, a recent work illustrated that dihydroartemisinin (DHA) could promote ferroptosis against tumoral cells, particularly in the presence of ferrous compounds. To achieve combined chemotherapy and ferroptosis, a nanocarrier (TKNPDHA-Fc) was constructed by using thioketal (TK)-bridged paclitaxel prodrug (PEG-TK-PTX) and ferrocene (Fc)-conjugated PEG-Fc, where DHA was encapsulated by a hydrophobic–hydrophobic interaction. Upon cellular uptake, TKNPDHA-Fc could facilitate PTX release through TK breakage under an excess H2O2 microenvironment. Owing to the loss of the hydrophobic PTX component, TKNPDHA-Fc underwent a rapid dissociation for improving DHA to act as a ferroptotic inducer along with Fe supplied from Fc. Moreover, both the chemotherapy-induced reactive oxygen species and the •OH produced from reinforced ferroptosis further stimulated the TK cleavage. The “self-catalytic” loop of TKNPDHA-Fc remarkably improved the antitumor performance in vivo via combined mechanisms, and its tumor inhibition rate reached 78.3%. This work highlights the contribution of ROS-responsive and self-catalytic nanoplatforms for enhancing the potential of combined chemotherapy and ferroptosis for cancer therapy in the future.
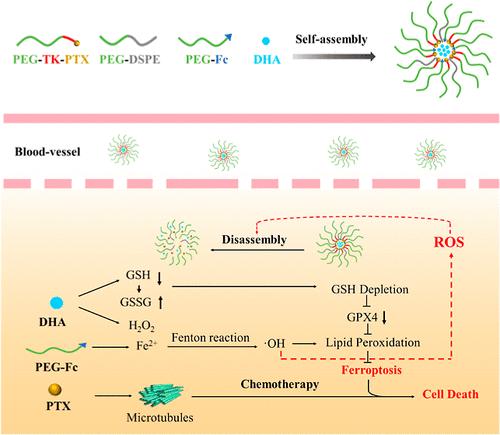
具有 ROS 响应性和自催化性的纳米载体,可将化疗和强化铁突变结合起来对抗乳腺癌
铁氧化是一种很有吸引力的癌症治疗策略,它基于 H2O2 参与的 Fenton 反应,产生有毒的 -OH 以促进脂质过氧化。然而,细胞内的 H2O2 很容易被消耗,导致芬顿反应不充分。传统的化疗药物可以通过补充 H2O2 来克服这一障碍。此外,最近的一项研究表明,双氢青蒿素(DHA)可以促进肿瘤细胞的铁变态反应,尤其是在亚铁化合物存在的情况下。为了实现化疗与铁凋亡的结合,研究人员利用硫酮(TK)杂化的紫杉醇原药(PEG-TK-PTX)和二茂铁(Fc)共轭的 PEG-Fc 构建了一种纳米载体(TKNPDHA-Fc),其中 DHA 通过疏水-疏水相互作用被包裹。细胞吸收后,TKNPDHA-Fc 可在过量 H2O2 的微环境下通过 TK 断裂促进 PTX 释放。由于失去了疏水性的 PTX 成分,TKNPDHA-Fc 发生了快速解离,从而改善了 DHA,使其与 Fc 提供的铁一起充当铁变态反应诱导剂。此外,化疗诱导的活性氧和强化铁凋亡产生的-OH都进一步刺激了TK的裂解。TKNPDHA-Fc的 "自催化 "环通过联合机制显著提高了体内的抗肿瘤性能,其肿瘤抑制率达到78.3%。这项工作凸显了ROS响应和自催化纳米平台在未来提高化疗和铁氧体结合治疗癌症潜力方面的贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Biomaterials Science & Engineering
Materials Science-Biomaterials
CiteScore
10.30
自引率
3.40%
发文量
413
期刊介绍:
ACS Biomaterials Science & Engineering is the leading journal in the field of biomaterials, serving as an international forum for publishing cutting-edge research and innovative ideas on a broad range of topics:
Applications and Health – implantable tissues and devices, prosthesis, health risks, toxicology
Bio-interactions and Bio-compatibility – material-biology interactions, chemical/morphological/structural communication, mechanobiology, signaling and biological responses, immuno-engineering, calcification, coatings, corrosion and degradation of biomaterials and devices, biophysical regulation of cell functions
Characterization, Synthesis, and Modification – new biomaterials, bioinspired and biomimetic approaches to biomaterials, exploiting structural hierarchy and architectural control, combinatorial strategies for biomaterials discovery, genetic biomaterials design, synthetic biology, new composite systems, bionics, polymer synthesis
Controlled Release and Delivery Systems – biomaterial-based drug and gene delivery, bio-responsive delivery of regulatory molecules, pharmaceutical engineering
Healthcare Advances – clinical translation, regulatory issues, patient safety, emerging trends
Imaging and Diagnostics – imaging agents and probes, theranostics, biosensors, monitoring
Manufacturing and Technology – 3D printing, inks, organ-on-a-chip, bioreactor/perfusion systems, microdevices, BioMEMS, optics and electronics interfaces with biomaterials, systems integration
Modeling and Informatics Tools – scaling methods to guide biomaterial design, predictive algorithms for structure-function, biomechanics, integrating bioinformatics with biomaterials discovery, metabolomics in the context of biomaterials
Tissue Engineering and Regenerative Medicine – basic and applied studies, cell therapies, scaffolds, vascularization, bioartificial organs, transplantation and functionality, cellular agriculture
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


