A Comprehensive Study of Density, Viscosity, and Electrical Conductivity of Choline Halide-Based Eutectic Solvents in H2O
IF 2.1
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This work focused on how the addition of H2O influences the properties of eutectic solvents with different anions and hydroxyl group numbers. Choline halides (ChCl, ChBr, and ChI) were chosen as the hydrogen bond acceptors, while ethylene glycol (EG) and glycerol (Gly) acted as the hydrogen bond donors at a 1:2 molar ratio. The density and viscosity measurements were conducted for four out of six systems, specifically those containing Br– and I–, across appropriate temperature and concentration ranges. Moreover, electrical conductivities were measured for all six systems. The excess molar volume and viscosity deviation were obtained and combined with those of (ChCl/EG + H2O) and (ChCl/Gly + H2O) for further analysis. The excess molar volume and the viscosity deviation both indicate that the contribution of H-bonding interactions is greater than packing effects, and the strengths of the H-bonding interaction are in the orders of Cl– > Br– > I– and Gly > EG. Under the competition of ion concentration, viscosity, and ion interaction, the specific conductivity of the eutectic solvent solution first increases to a maximum and then decreases.
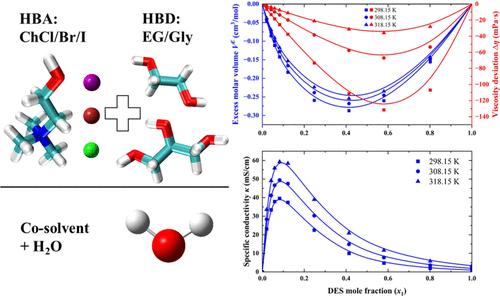
卤化胆碱共晶溶剂在 H2O 中的密度、粘度和导电性综合研究
这项工作的重点是研究加入 H2O 如何影响具有不同阴离子和羟基数的共晶溶剂的性质。我们选择胆碱卤化物(ChCl、ChBr 和 ChI)作为氢键受体,而乙二醇(EG)和甘油(Gly)则以 1:2 的摩尔比作为氢键供体。在适当的温度和浓度范围内,对六个体系中的四个,特别是含有 Br- 和 I- 的体系进行了密度和粘度测量。此外,还测量了所有六种体系的电导率。获得了过量摩尔体积和粘度偏差,并将其与(ChCl/EG + H2O)和(ChCl/Gly + H2O)的过量摩尔体积和粘度偏差结合起来作进一步分析。过剩摩尔体积和粘度偏差均表明,H 键相互作用的贡献大于填料效应,H 键相互作用的强度依次为 Cl- > Br- > I- 和 Gly > EG。在离子浓度、粘度和离子相互作用的竞争下,共晶溶剂溶液的比电导率先上升到最大值,然后下降。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


